Abstract
Crit Care Sci. 2023;35(4):345-354
DOI 10.5935/2965-2774.20230162-pt
The optimal target for blood glucose concentration in critically ill patients is unclear. We will perform a systematic review and meta-analysis with aggregated and individual patient data from randomized controlled trials, comparing intensive glucose control with liberal glucose control in critically ill adults.
MEDLINE®, Embase, the Cochrane Central Register of Clinical Trials, and clinical trials registries (World Health Organization, clinical trials.gov). The authors of eligible trials will be invited to provide individual patient data. Published trial-level data from eligible trials that are not at high risk of bias will be included in an aggregated data meta-analysis if individual patient data are not available.
Inclusion criteria: randomized controlled trials that recruited adult patients, targeting a blood glucose of ≤ 120mg/dL (≤ 6.6mmol/L) compared to a higher blood glucose concentration target using intravenous insulin in both groups. Excluded studies: those with an upper limit blood glucose target in the intervention group of > 120mg/dL (> 6.6mmol/L), or where intensive glucose control was only performed in the intraoperative period, and those where loss to follow-up exceeded 10% by hospital discharge.
In-hospital mortality during index hospital admission. Secondary endpoints: mortality and survival at other timepoints, duration of invasive mechanical ventilation, vasoactive agents, and renal replacement therapy. A random effect Bayesian meta-analysis and hierarchical Bayesian models for individual patient data will be used.
This systematic review with aggregate and individual patient data will address the clinical question, ‘what is the best blood glucose target for critically ill patients overall?’
Abstract
Crit Care Sci. 2023;35(3):256-265
DOI 10.5935/2965-2774.20230129-pt
Critical illness is a major ongoing health care burden worldwide and is associated with high mortality rates. Sodium-glucose cotransporter-2 inhibitors have consistently shown benefits in cardiovascular and renal outcomes. The effects of sodium-glucose cotransporter-2 inhibitors in acute illness have not been properly investigated.
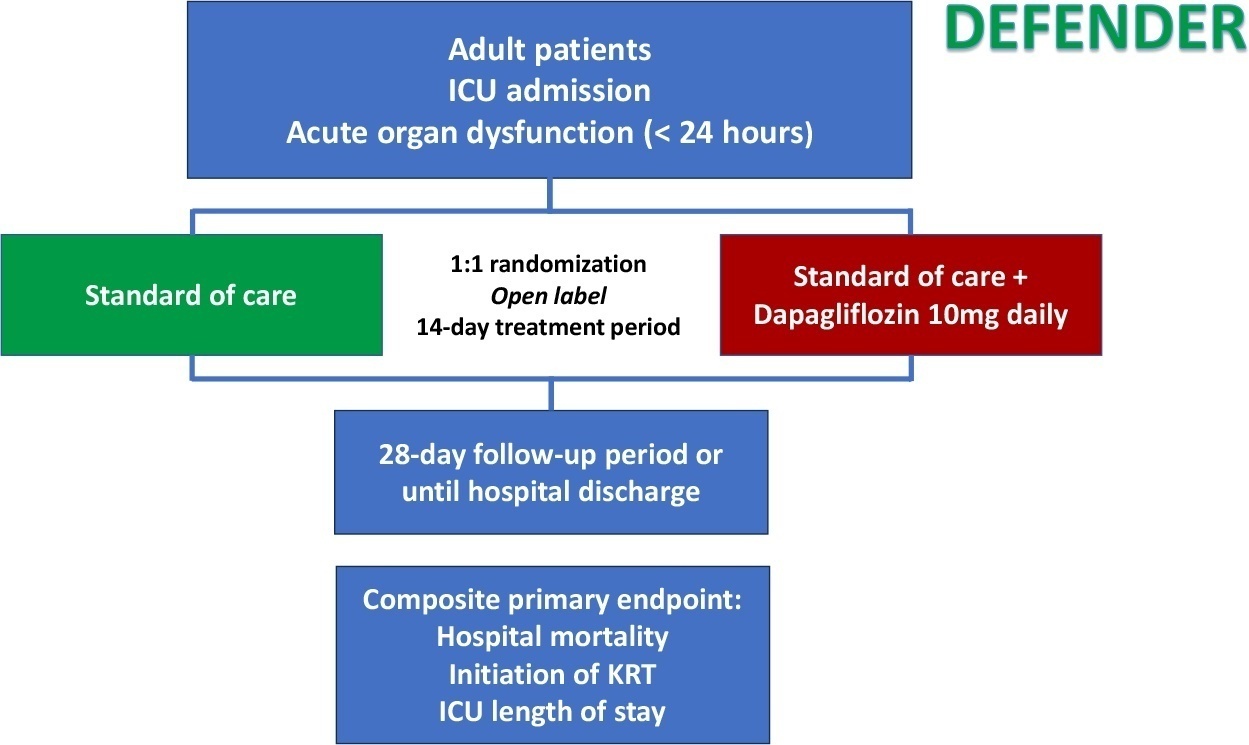
DEFENDER is an investigator-initiated, multicenter, randomized, open-label trial designed to evaluate the efficacy and safety of dapagliflozin in 500 adult participants with acute organ dysfunction who are hospitalized in the intensive care unit. Eligible participants will be randomized 1:1 to receive dapagliflozin 10mg plus standard of care for up to 14 days or standard of care alone. The primary outcome is a hierarchical composite of hospital mortality, initiation of kidney replacement therapy, and intensive care unit length of stay, up to 28 days. Safety will be strictly monitored throughout the study.
DEFENDER is the first study designed to investigate the use of a sodium-glucose cotransporter-2 inhibitor in general intensive care unit patients with acute organ dysfunction. It will provide relevant information on the use of drugs of this promising class in critically ill patients.
NCT05558098
Abstract
Crit Care Sci. 2023;35(2):117-146
DOI 10.5935/2965-2774.20230310-pt
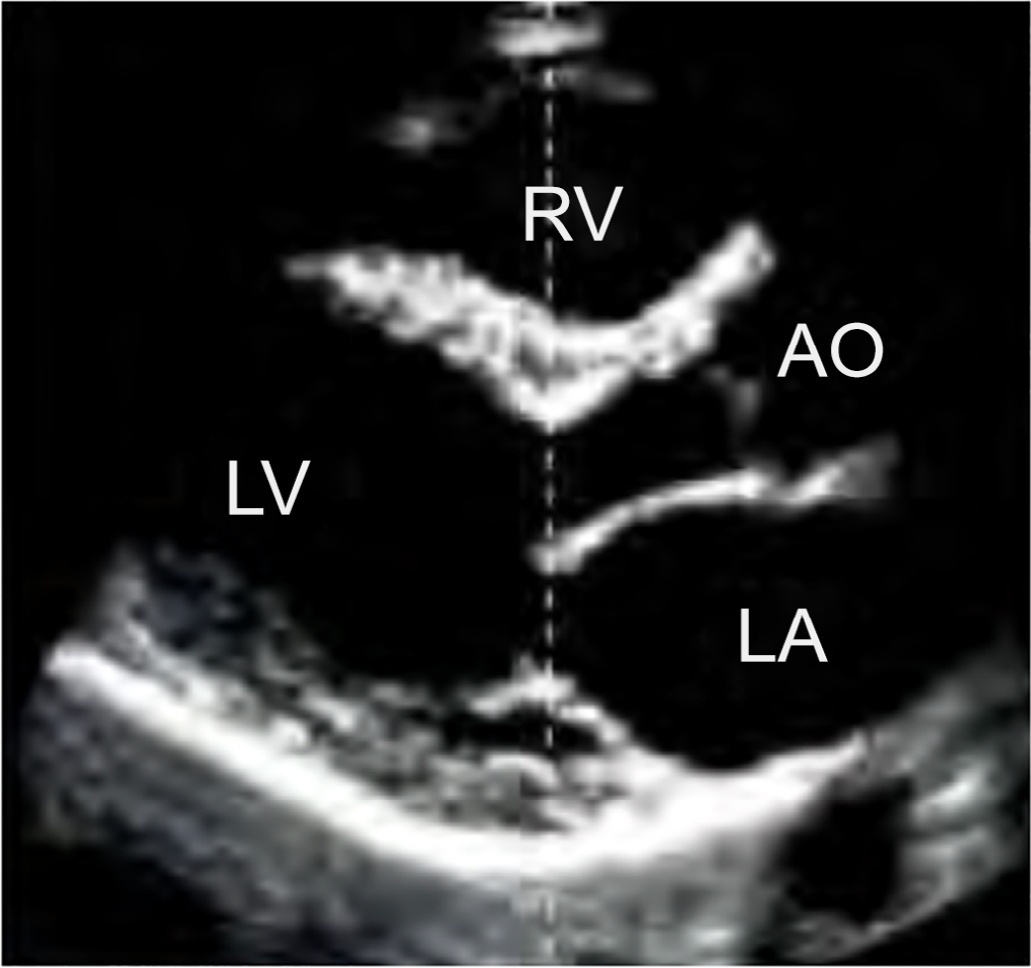
Echocardiography in critically ill patients has become essential in the evaluation of patients in different settings, such as the hospital. However, unlike for other matters related to the care of these patients, there are still no recommendations from national medical societies on the subject. The objective of this document was to organize and make available expert consensus opinions that may help to better incorporate echocardiography in the evaluation of critically ill patients. Thus, the Associação de Medicina Intensiva Brasileira, the Associação Brasileira de Medicina de Emergência, and the Sociedade Brasileira de Medicina Hospitalar formed a group of 17 physicians to formulate questions relevant to the topic and discuss the possibility of consensus for each of them. All questions were prepared using a five-point Likert scale. Consensus was defined a priori as at least 80% of the responses between one and two or between four and five. The consideration of the issues involved two rounds of voting and debate among all participants. The 27 questions prepared make up the present document and are divided into 4 major assessment areas: left ventricular function, right ventricular function, diagnosis of shock, and hemodynamics. At the end of the process, there were 17 positive (agreement) and 3 negative (disagreement) consensuses; another 7 questions remained without consensus. Although areas of uncertainty persist, this document brings together consensus opinions on several issues related to echocardiography in critically ill patients and may enhance its development in the national scenario.
Abstract
Crit Care Sci. 2023;35(2):147-155
DOI 10.5935/2965-2774.20230422-pt
To assess factors associated with long-term neuropsychiatric outcomes, including biomarkers measured after discharge from the intensive care unit.
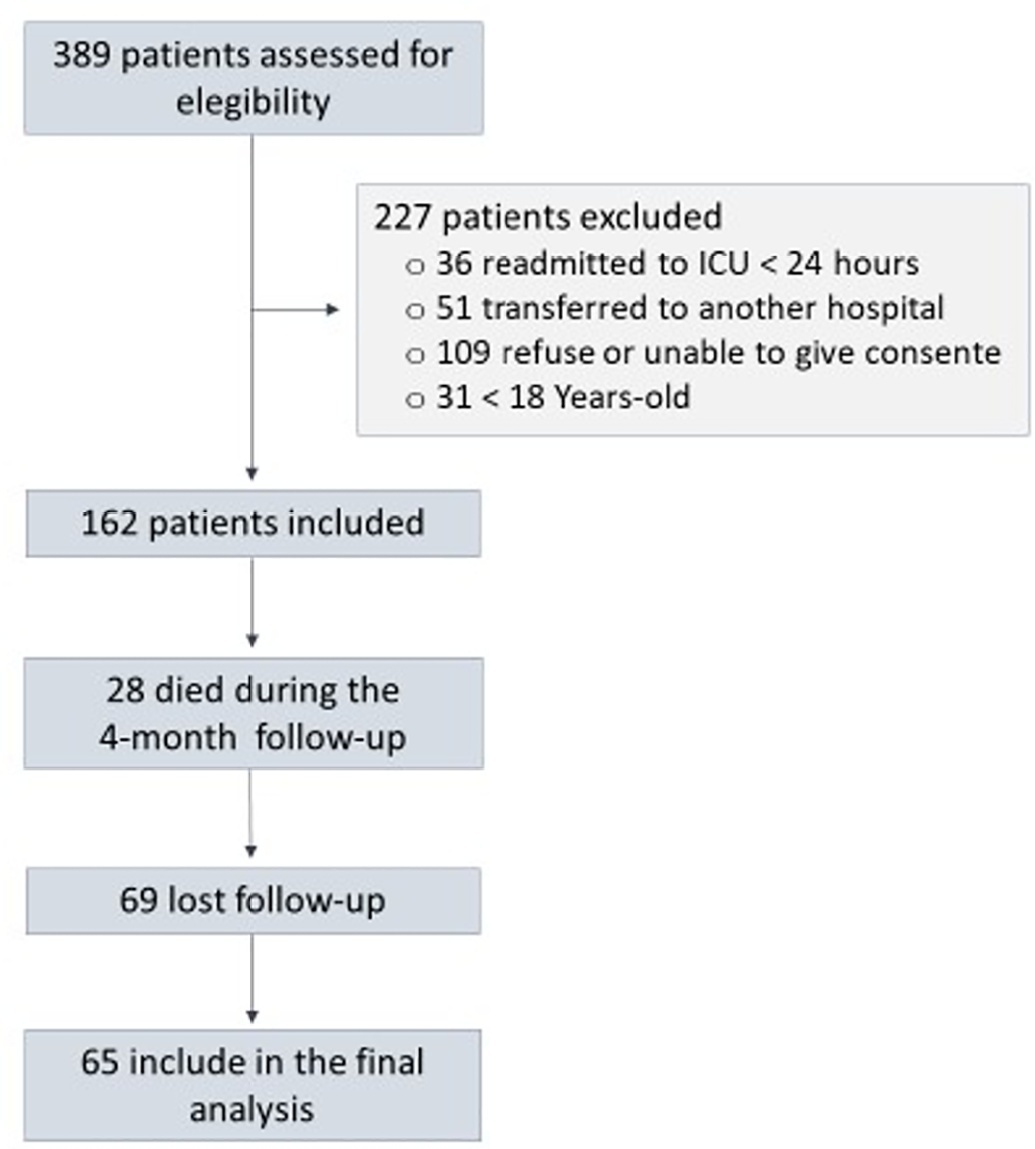
A prospective cohort study was performed with 65 intensive care unit survivors. The cognitive evaluation was performed through the Mini-Mental State Examination, the symptoms of anxiety and depression were evaluated using the Hospital Anxiety and Depression Scale, and posttraumatic stress disorder was evaluated using the Impact of Event Scale-6. Plasma levels of amyloid-beta (1-42) [Aβ (1-42)], Aβ (1-40), interleukin (IL)-10, IL-6, IL-33, IL-4, IL-5, tumor necrosis factor alpha, C-reactive protein, and brain-derived neurotrophic factor were measured at intensive care unit discharge.
Of the variables associated with intensive care, only delirium was independently related to the occurrence of long-term cognitive impairment. In addition, higher levels of IL-10 and IL-6 were associated with cognitive dysfunction. Only IL-6 was independently associated with depression. Mechanical ventilation, IL-33 levels, and C-reactive protein levels were independently associated with anxiety. No variables were independently associated with posttraumatic stress disorder.
Cognitive dysfunction, as well as symptoms of depression, anxiety, and posttraumatic stress disorder, are present in patients who survive a critical illness, and some of these outcomes are associated with the levels of inflammatory biomarkers measured at discharge from the intensive care unit.
Abstract
Crit Care Sci. 2023;35(1):84-96
DOI 10.5935/2965-2774.20230405-pt
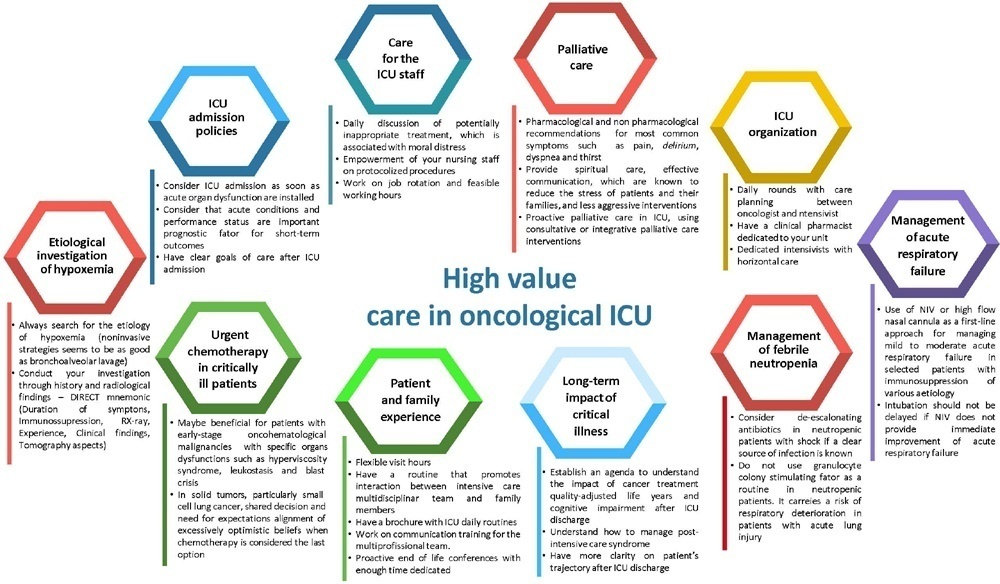
The number of patients with cancer requiring intensive care unit admission is increasing around the world. The improvement in the pathophysiological understanding of this group of patients, as well as the increasingly better and more targeted treatment options for their underlying disease, has led to a significant increase in their survival over the past three decades. Within the organizational concepts, it is necessary to know what adds value in the care of critical oncohematological patients. Practices in medicine that do not benefit patients and possibly cause harm are called low-value practices, while high-value practices are defined as high-quality care at relatively low cost. In this article, we discuss ten domains with high-value evidence in the care of cancer patients: (1) intensive care unit admission policies; (2) intensive care unit organization; (3) etiological investigation of hypoxemia; (4) management of acute respiratory failure; (5) management of febrile neutropenia; (6) urgent chemotherapy treatment in critically ill patients; (7) patient and family experience; (8) palliative care; (9) care of intensive care unit staff; and (10) long-term impact of critical disease on the cancer population. The disclosure of such policies is expected to have the potential to change health care standards. We understand that it is a lengthy process, and initiatives such as this paper are one of the first steps in raising awareness and beginning a discussion about high-value care in various health scenarios.
Abstract
Crit Care Sci. 2023;35(1):44-56
DOI 10.5935/2965-2774.20230340-pt
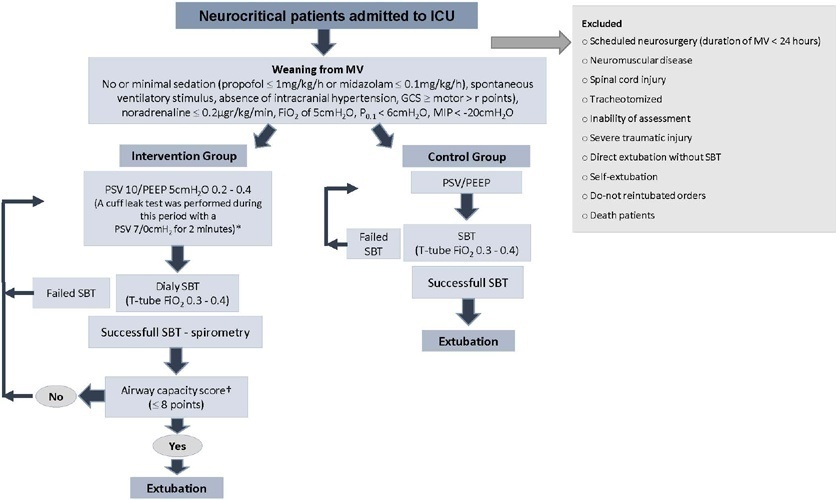
To investigate whether protocol-directed weaning in neurocritical patients would reduce the rate of extubation failure (as a primary outcome) and the associated complications (as a secondary outcome) compared with conventional weaning.
A quasi-experimental study was conducted in a medical-surgical intensive care unit from January 2016 to December 2018. Patients aged 18 years or older with an acute neurological disease who were on mechanical ventilation > 24 hours were included. All patients included in the study were ready to wean, with no or minimal sedation, Glasgow coma score ≥ 9, spontaneous ventilatory stimulus, noradrenaline ≤ 0.2μgr/kg/ minute, fraction of inspired oxygen ≤ 0.5, positive end-expiratory pressure ≤ 5cmH2O, maximal inspiratory pressure < -20cmH2O, and occlusion pressure < 6cmH2O.
Ninety-four of 314 patients admitted to the intensive care unit were included (50 in the Intervention Group and 44 in the Control Group). There was no significant difference in spontaneous breathing trial failure (18% in the Intervention Group versus 34% in the Control Group, p = 0.12). More patients in the Intervention Group were extubated than in the Control Group (100% versus 79%, p = 0.01). The rate of extubation failure was not signifiantly diffrent between the groups (18% in the Intervention Group versus 17% in the Control Group; relative risk 1.02; 95%CI 0.64 - 1.61; p = 1.00). The reintubation rate was lower in the Control Group (16% in the Intervention Group versus 11% in the Control Group; relative risk 1.15; 95%CI 0.74 - 1.82; p = 0.75). The need for tracheotomy was lower in the Intervention Group [4 (8%) versus 11 (25%) in the Control Group; relative risk 0.32; 95%CI 0.11 - 0.93; p = 0.04]. At Day 28, the patients in the Intervention Group had more ventilator-free days than those in the Control Group [28 (26 - 28) days versus 26 (19 - 28) days; p = 0.01]. The total duration of mechanical ventilation was shorter in the Intervention Group than in the Control Group [5 (2 - 13) days versus 9 (3 - 22) days; p = 0.01]. There were no diffrences in the length of intensive care unit stay, 28-day free from mechanical ventilation, hospital stay or 90-day mortality.
Considering the limitations of our study, the application of a weaning protocol for neurocritical patients led to a high percentage of extubation, a reduced need for tracheotomy and a shortened duration of mechanical ventilation. However, there was no reduction in extubation failure or the 28-day free of from mechanical ventilation compared with the Control Group.
Abstract
Crit Care Sci. 2023;35(1):73-83
DOI 10.5935/2965-2774.20230374-pt
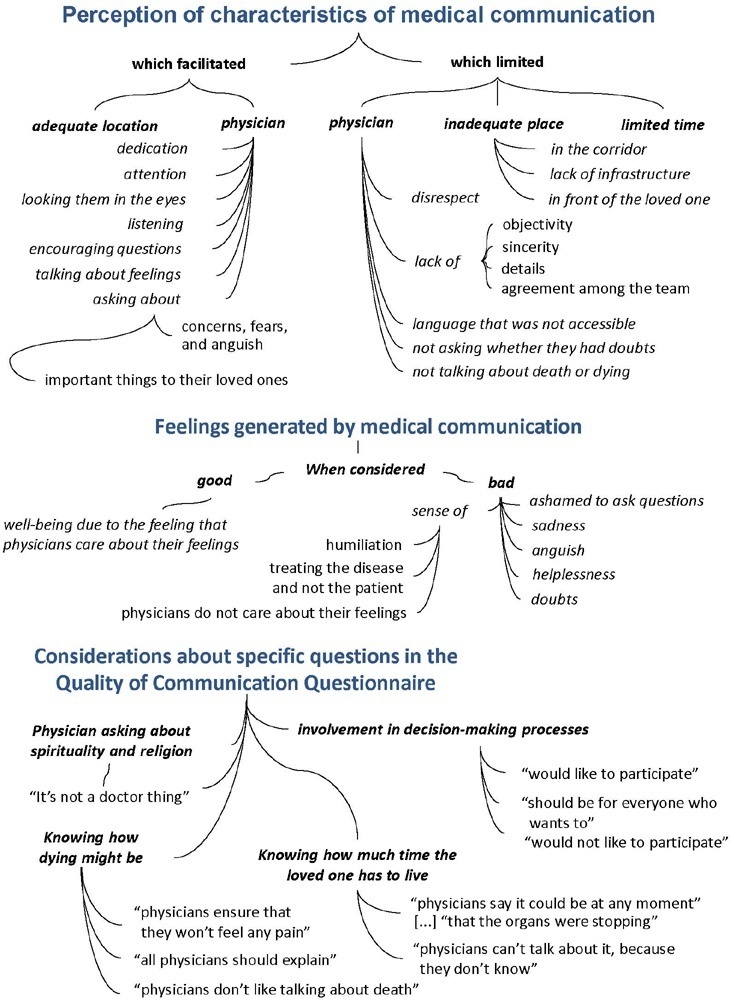
To understand the perception of medical communication and needs of family members with loved ones in intensive care.
The study was mainly qualitative and exploratory, with thematic analysis of comments made by 92 family members with loved ones in intensive care units when answering in-person interviews comprising the Quality of Communication Questionnaire (QoC) and open-ended questions about their need for additional help, the appropriateness of the place where they received information, and additional comments.
The participants’ mean age was 46.8 years (SD = 11.8), and most of them were female, married and had incomplete or completed elementary education. The following themes were found: perception of characteristics of medical communication; feelings generated by communication; considerations about specific questions in the QoC; family members’ needs; and strategies to overcome needs regarding communication. Characteristics that facilitated communication included attention and listening. Characteristics that made communication difficult included aspects of information sharing, such as inaccessible language; lack of clarity, objectivity, sincerity, and agreement among the team; limited time; and inadequate location. Feelings such as shame, helplessness, and sadness were cited when communication was inadequate. Family members’ needs related to communication included more details about the loved one’s diagnosis, prognosis, and health condition; participation in decisionmaking; and being asked about feelings, spirituality, dying and death. Others were related to longer visitation time, psychological support, social assistance, and better infrastructure.
It is necessary to enhance medical communication and improve hospital infrastructure to improve the quality of care for family members.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (33) COVID-19 (45) Critical care (115) Critical illness (54) ICU (25) Infant, newborn (27) Intensive care (72) Intensive care units (254) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (75) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (117) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)