Abstract
Crit Care Sci. 2023;35(4):345-354
DOI 10.5935/2965-2774.20230162-pt
The optimal target for blood glucose concentration in critically ill patients is unclear. We will perform a systematic review and meta-analysis with aggregated and individual patient data from randomized controlled trials, comparing intensive glucose control with liberal glucose control in critically ill adults.
MEDLINE®, Embase, the Cochrane Central Register of Clinical Trials, and clinical trials registries (World Health Organization, clinical trials.gov). The authors of eligible trials will be invited to provide individual patient data. Published trial-level data from eligible trials that are not at high risk of bias will be included in an aggregated data meta-analysis if individual patient data are not available.
Inclusion criteria: randomized controlled trials that recruited adult patients, targeting a blood glucose of ≤ 120mg/dL (≤ 6.6mmol/L) compared to a higher blood glucose concentration target using intravenous insulin in both groups. Excluded studies: those with an upper limit blood glucose target in the intervention group of > 120mg/dL (> 6.6mmol/L), or where intensive glucose control was only performed in the intraoperative period, and those where loss to follow-up exceeded 10% by hospital discharge.
In-hospital mortality during index hospital admission. Secondary endpoints: mortality and survival at other timepoints, duration of invasive mechanical ventilation, vasoactive agents, and renal replacement therapy. A random effect Bayesian meta-analysis and hierarchical Bayesian models for individual patient data will be used.
This systematic review with aggregate and individual patient data will address the clinical question, ‘what is the best blood glucose target for critically ill patients overall?’
Abstract
Crit Care Sci. 2023;35(3):243-255
DOI 10.5935/2965-2774.20230136-pt
To update the recommendations to support decisions regarding the pharmacological treatment of patients hospitalized with COVID-19 in Brazil.
Experts, including representatives of the Ministry of Health and methodologists, created this guideline. The method used for the rapid development of guidelines was based on the adoption and/or adaptation of existing international guidelines (GRADE ADOLOPMENT) and supported by the e-COVID-19 RecMap platform. The quality of the evidence and the preparation of the recommendations followed the GRADE method.
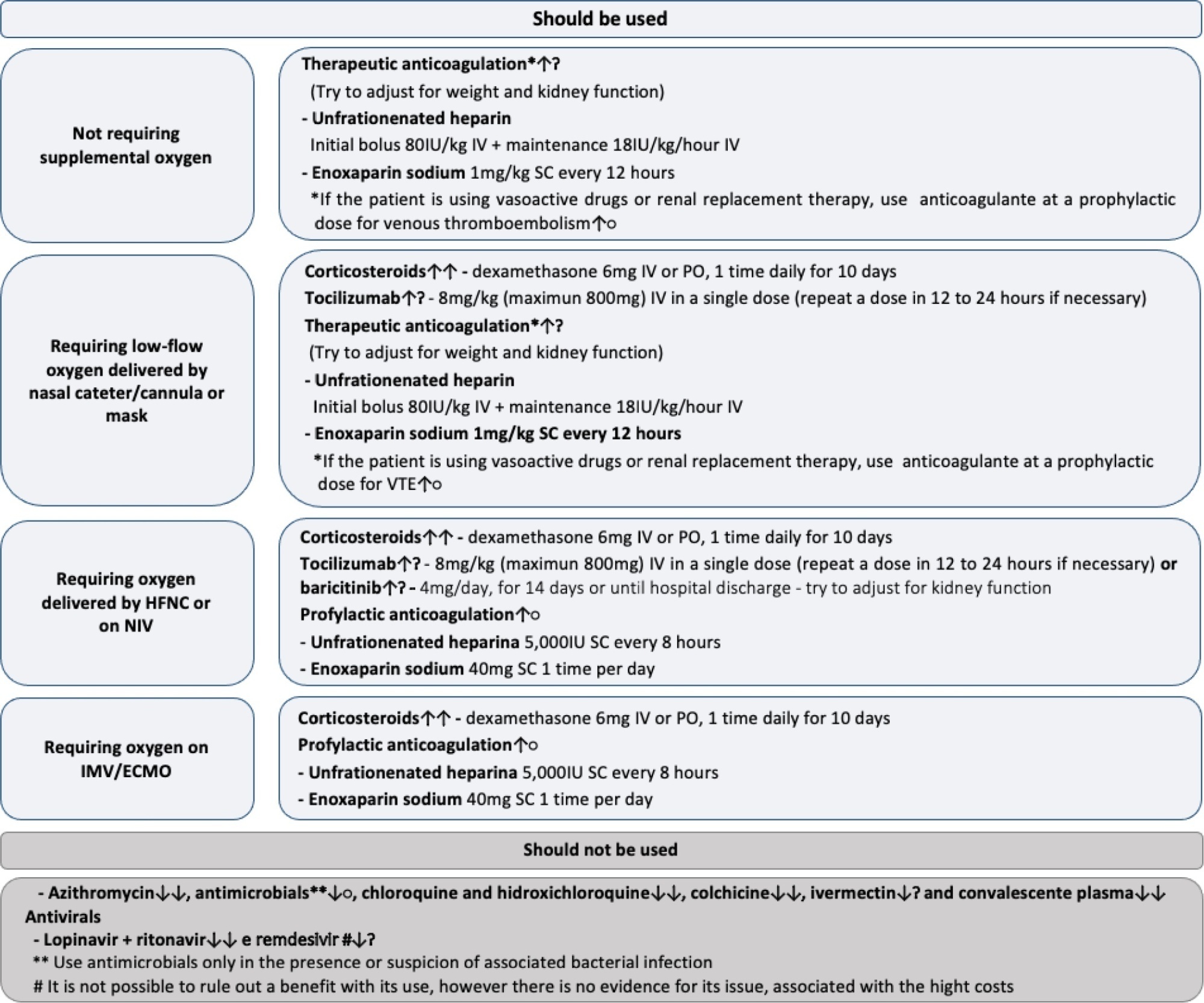
Twenty-one recommendations were generated, including strong recommendations for the use of corticosteroids in patients using supplemental oxygen and conditional recommendations for the use of tocilizumab and baricitinib for patients on supplemental oxygen or on noninvasive ventilation and anticoagulants to prevent thromboembolism. Due to suspension of use authorization, it was not possible to make recommendations regarding the use of casirivimab + imdevimab. Strong recommendations against the use of azithromycin in patients without suspected bacterial infection, hydroxychloroquine, convalescent plasma, colchicine, and lopinavir + ritonavir and conditional recommendations against the use of ivermectin and remdesivir were made.
New recommendations for the treatment of hospitalized patients with COVID-19 were generated, such as those for tocilizumab and baricitinib. Corticosteroids and prophylaxis for thromboembolism are still recommended, the latter with conditional recommendation. Several drugs were considered ineffective and should not be used to provide the best treatment according to the principles of evidence-based medicine and to promote resource economy.
Abstract
Crit Care Sci. 2023;35(3):256-265
DOI 10.5935/2965-2774.20230129-pt
Critical illness is a major ongoing health care burden worldwide and is associated with high mortality rates. Sodium-glucose cotransporter-2 inhibitors have consistently shown benefits in cardiovascular and renal outcomes. The effects of sodium-glucose cotransporter-2 inhibitors in acute illness have not been properly investigated.
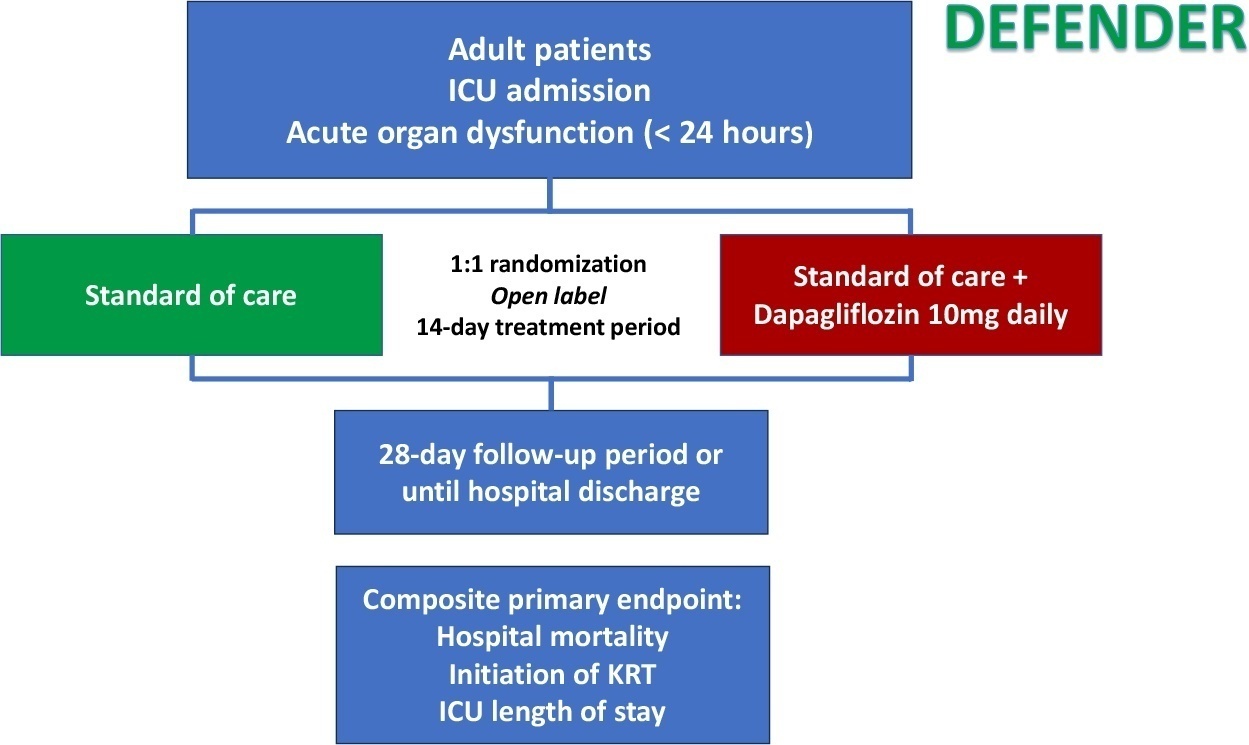
DEFENDER is an investigator-initiated, multicenter, randomized, open-label trial designed to evaluate the efficacy and safety of dapagliflozin in 500 adult participants with acute organ dysfunction who are hospitalized in the intensive care unit. Eligible participants will be randomized 1:1 to receive dapagliflozin 10mg plus standard of care for up to 14 days or standard of care alone. The primary outcome is a hierarchical composite of hospital mortality, initiation of kidney replacement therapy, and intensive care unit length of stay, up to 28 days. Safety will be strictly monitored throughout the study.
DEFENDER is the first study designed to investigate the use of a sodium-glucose cotransporter-2 inhibitor in general intensive care unit patients with acute organ dysfunction. It will provide relevant information on the use of drugs of this promising class in critically ill patients.
NCT05558098
Abstract
Crit Care Sci. 2023;35(3):266-272
DOI 10.5935/2965-2774.20230223-pt
The objective of this study is to present the protocol of a cluster randomized clinical trial to be conducted through the TeleICU project - Qualification of Intensive Care by Telemedicine. The study will consist of a cluster randomized clinical trial, open label, in pediatric intensive care units, with an allocation ratio of 1:1, to compare the intervention group (support of Telemedicine for patients admitted to the pediatric intensive care unit) with a control group (pediatric intensive care unit usual care). The study proposed to select 16 pediatric intensive care units, including 100 participants per site, with a total of 1,600 participants. The intervention group will receive telerounds from Monday to Friday and will have specialists and continuing education activities available. The primary outcome measure will be the length of stay in the pediatric intensive care unit, defined as the difference between the date of discharge of the participant and the date of admission to the intensive care unit. The secondary outcomes will be mortality rate, invasive mechanical ventilation-free days, days using antibiotics, days using vasoactive drugs and days using sedoanalgesia. This study will be conducted in accordance with Resolution 466/12 of the National Health Council, with approval by the Research Ethics Committee of the institutions involved. The present study has the potential to reproduce studies on Telemedicine in intensive care and may make important contributions to care in intensive care units in Brazil and other settings. If Telemedicine shows positive clinical care results compared to conventional treatment, more pediatric patients may benefit.
ClinicalTrials.gov registry: NCT05260710
Abstract
Crit Care Sci. 2023;35(2):117-146
DOI 10.5935/2965-2774.20230310-pt
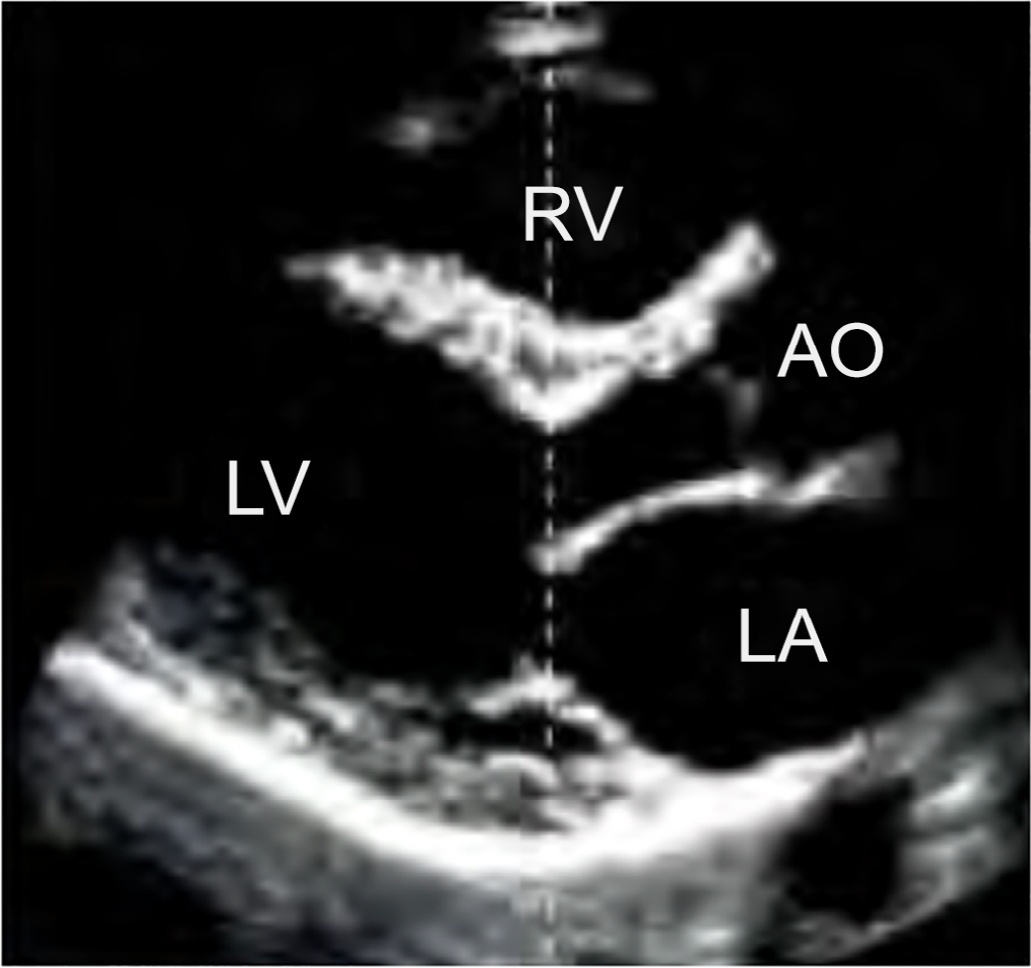
Echocardiography in critically ill patients has become essential in the evaluation of patients in different settings, such as the hospital. However, unlike for other matters related to the care of these patients, there are still no recommendations from national medical societies on the subject. The objective of this document was to organize and make available expert consensus opinions that may help to better incorporate echocardiography in the evaluation of critically ill patients. Thus, the Associação de Medicina Intensiva Brasileira, the Associação Brasileira de Medicina de Emergência, and the Sociedade Brasileira de Medicina Hospitalar formed a group of 17 physicians to formulate questions relevant to the topic and discuss the possibility of consensus for each of them. All questions were prepared using a five-point Likert scale. Consensus was defined a priori as at least 80% of the responses between one and two or between four and five. The consideration of the issues involved two rounds of voting and debate among all participants. The 27 questions prepared make up the present document and are divided into 4 major assessment areas: left ventricular function, right ventricular function, diagnosis of shock, and hemodynamics. At the end of the process, there were 17 positive (agreement) and 3 negative (disagreement) consensuses; another 7 questions remained without consensus. Although areas of uncertainty persist, this document brings together consensus opinions on several issues related to echocardiography in critically ill patients and may enhance its development in the national scenario.
Abstract
Crit Care Sci. 2023;35(1):2-10
DOI 10.5935/2965-2774.20230307-pt
The use of echocardiography by physicians who are not echocardiographers has become common throughout the world across highly diverse settings where the care of acutely ill patients is provided. Echocardiographic evaluation performed in a point-of-care manner can provide relevant information regarding the mechanism of causes of shock, for example, increasing the rates of correct diagnosis and allowing for faster informed decision-making than through evaluation methods. Considering that the accurate diagnosis of life-threatening situations is essential for professionals working with acutely ill patients, several international associations recommend that physicians responsible for critically ill patients acquire and develop the ability to perform bedside ultrasound examinations, including echocardiographic examinations. However, there is no consensus in the literature regarding which specific applications should be included in the list of skills for nonechocardiographer physicians. Taking into account the multiplicity of applications of echocardiography in different scenarios related to acutely ill patients; the differences in the published protocols, with regard to both the teaching methodology and competence verification; and the heterogeneity of training among highly diverse specialties responsible for their care at different levels, this consensus document aimed to reflect the position of representatives of related Brazilian medical societies on the subject and may thus serve as a starting point both for standardization among different specialties and for the transmission of knowledge and verification of the corresponding competencies.
Abstract
Crit Care Sci. 2023;35(1):11-18
DOI 10.5935/2965-2774.20230336-pt
To explain the rationale and protocol of the methods and analyses to be used in the LIVER-PAM randomized clinical trial, which seeks to understand whether a higher mean arterial pressure is capable of reducing the incidence of renal dysfunction postoperatively after liver transplantation.
LIVER-PAM is an open-label, randomized, controlled, singlecenter clinical trial. Patients randomized to the intervention group will have a mean arterial pressure of 85 - 90mmHg in the initial 24 hours of postoperative management, while patients in the control group will have a mean arterial pressure of 65 - 70mmHg in the same period. A sample of 174 patients will be required to demonstrate a 20% reduction in the absolute incidence of renal dysfunction, with a power of 80% and an alpha of 0.05.
If a 20% reduction in the absolute incidence of renal dysfunction in the postoperative period of liver transplantation is achieved with higher target mean arterial pressure in the first 24 hours, this would represent an inexpensive and simple therapy for improving current outcomes in the management of liver transplant patients.
Abstract
Rev Bras Ter Intensiva. 2022;34(3):319-326
DOI 10.5935/0103-507X.20220101-en
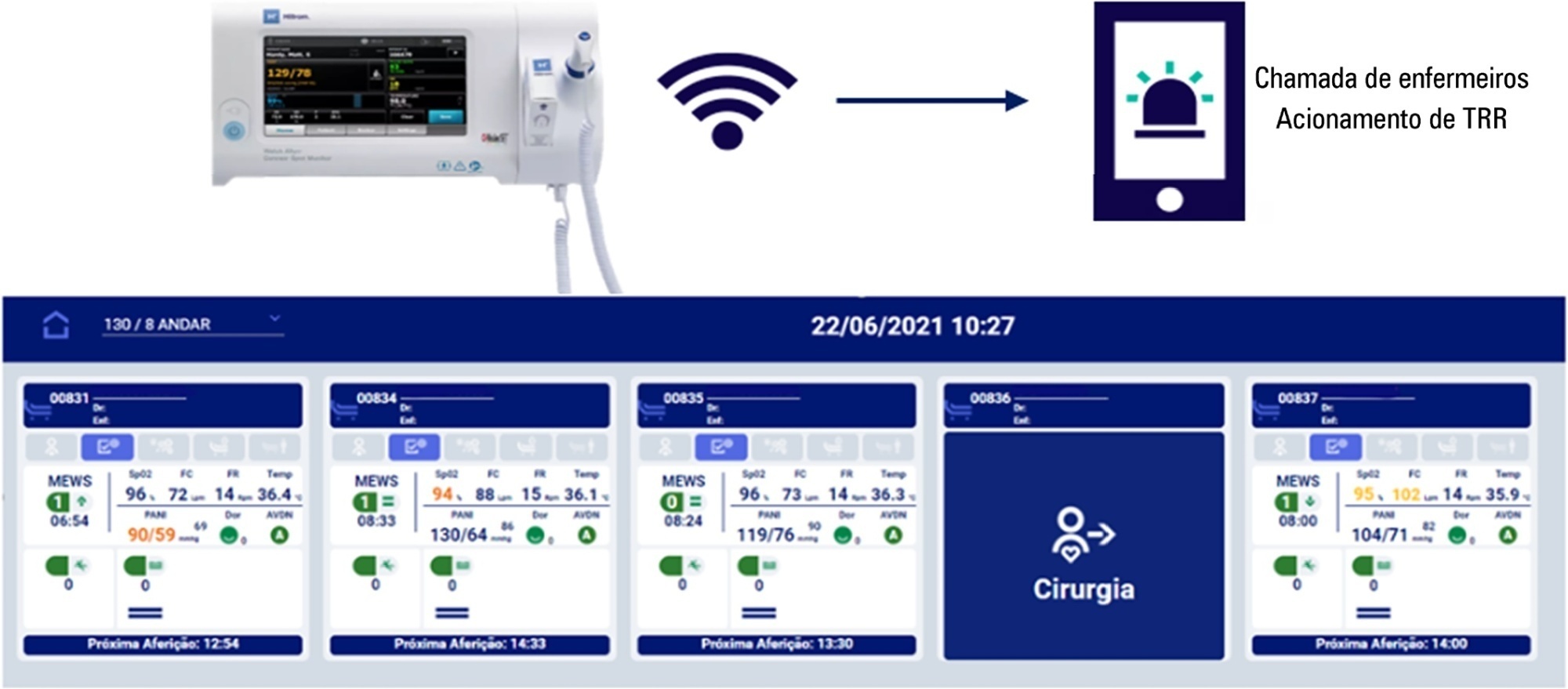
To evaluate the effectiveness of the Welch Allyn Connex® Spot Monitor/Hillrom Connecta™ solution in activating the rapid response team in a timely manner compared to manual activation.
The Hillrom study is a single-center, open-label, superiority, cluster-randomized, parallel-group (1:1 allocation ratio) clinical trial that will be conducted in a tertiary hospital. Two sets of three wards with 28 beds will be included (one as the intervention cluster and the other as the control). The wards will be randomly assigned to use the Welch Allyn Connex® Spot Monitor/Hillrom Connecta™ automated solution (intervention cluster) or to maintain the usual routine (control cluster) regarding rapid response team activation. The primary outcome will be the absolute number of episodes of rapid response team triggering in an appropriate time; as secondary outcomes, clinical features (mortality, cardiac arrest, need for intensive care unit admission and duration of hospitalization) will be assessed according to clusters in an exploratory way. A sample size of 216 rapid response team activations was estimated to identify a possible difference between the groups. The protocol has been approved by the institutional Research Ethics Committee.
The Welch Allyn Connex® Spot Monitor/Hillrom Connecta™ automated solution is expected to be more effective in triggering the nurse call system to activate the rapid response team in a timely and adequate manner compared to manual triggering (usual practice).
NCT04648579
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (33) COVID-19 (45) Critical care (115) Critical illness (54) Infant, newborn (27) Intensive care (72) Intensive care units (254) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (75) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (117) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98) Septic shock (25)