Abstract
Crit Care Sci. 2023;35(4):345-354
DOI 10.5935/2965-2774.20230162-pt
The optimal target for blood glucose concentration in critically ill patients is unclear. We will perform a systematic review and meta-analysis with aggregated and individual patient data from randomized controlled trials, comparing intensive glucose control with liberal glucose control in critically ill adults.
MEDLINE®, Embase, the Cochrane Central Register of Clinical Trials, and clinical trials registries (World Health Organization, clinical trials.gov). The authors of eligible trials will be invited to provide individual patient data. Published trial-level data from eligible trials that are not at high risk of bias will be included in an aggregated data meta-analysis if individual patient data are not available.
Inclusion criteria: randomized controlled trials that recruited adult patients, targeting a blood glucose of ≤ 120mg/dL (≤ 6.6mmol/L) compared to a higher blood glucose concentration target using intravenous insulin in both groups. Excluded studies: those with an upper limit blood glucose target in the intervention group of > 120mg/dL (> 6.6mmol/L), or where intensive glucose control was only performed in the intraoperative period, and those where loss to follow-up exceeded 10% by hospital discharge.
In-hospital mortality during index hospital admission. Secondary endpoints: mortality and survival at other timepoints, duration of invasive mechanical ventilation, vasoactive agents, and renal replacement therapy. A random effect Bayesian meta-analysis and hierarchical Bayesian models for individual patient data will be used.
This systematic review with aggregate and individual patient data will address the clinical question, ‘what is the best blood glucose target for critically ill patients overall?’
Abstract
Rev Bras Ter Intensiva. 2021;33(1):138-145
DOI 10.5935/0103-507X.20210015
To double the percentage of time within the 100 - 180mg/dL blood glucose range in the first three months following a phased implementation of a formal education program, and then, of an insulin therapy protocol, without entailing an increased incidence of hypoglycemia.
The pre-intervention glycemic control was assessed retrospectively. Next, were carried out the implementation of a formal education program, distribution of manual algorithms for intravenous insulin therapy - optimized by the users, based on the modified Yale protocol - and informal training of the nursing staff. The use of electronic blood glucose control systems was supported, and the results were recorded prospectively.
The first phase of the program (formal education) lead to improvement of the time within the euglycemic interval (28% to 37%). In the second phase, euglycemia was achieved 66% of the time, and the incidence of hypoglycemia was decreased. The percentage of patients on intravenous insulin infusion at 48 hours from admission increased from 6% to 35%.
The phased implementation of a formal education program, fostering the use of electronic insulin therapy protocols and dynamic manuals, received good adherence and has shown to be safe and effective for blood glucose control in critically ill patients, with a concomitant decrease in hypoglycemia.
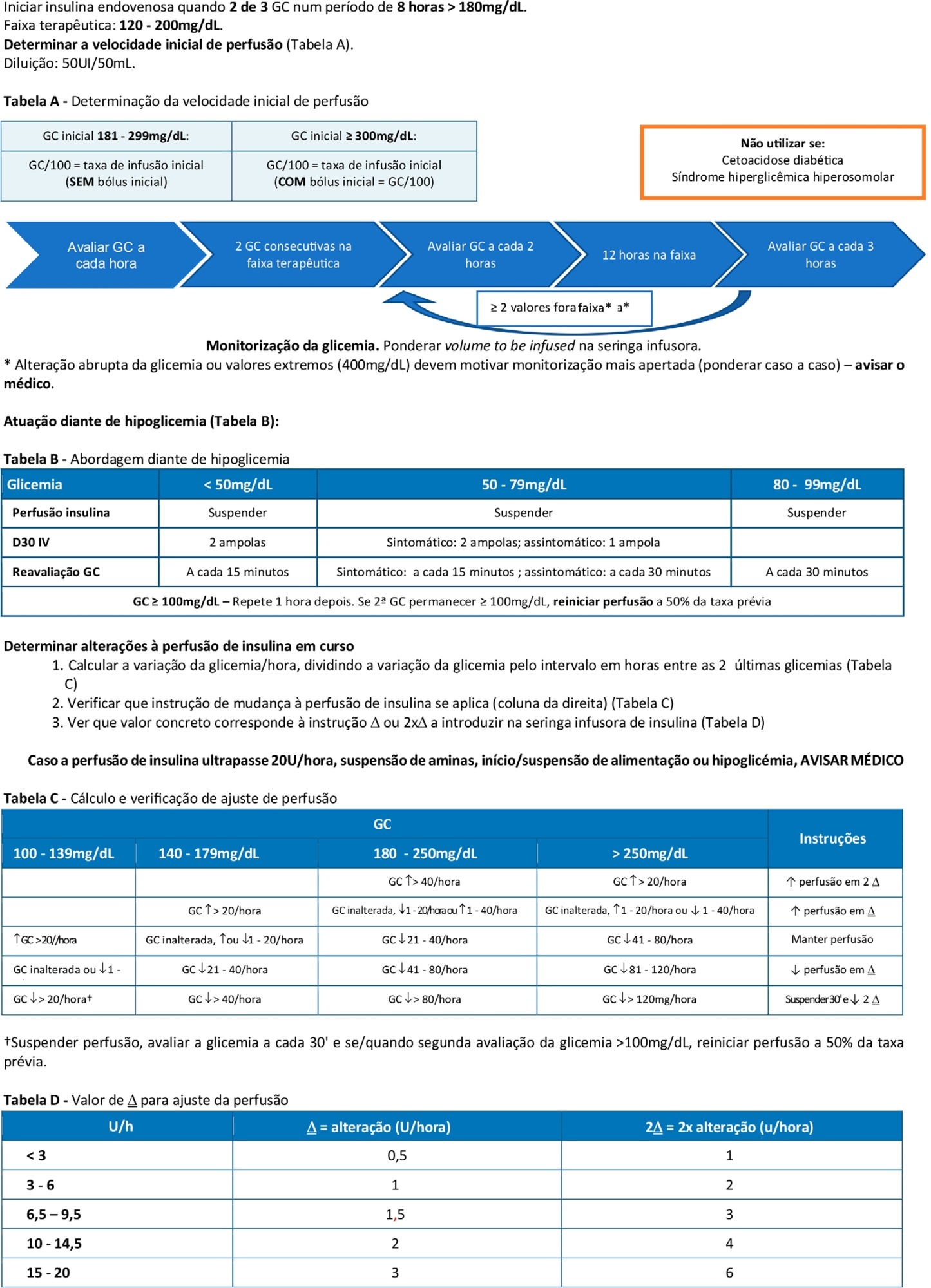
Abstract
Rev Bras Ter Intensiva. 2009;21(4):398-403
DOI 10.1590/S0103-507X2009000400010
OBJECTIVES: Stress-induced hyperglycemia is frequent in critically ill patients and has been associated with increased mortality and morbidity (both in diabetic and non-diabetic patients). This study objective was to evaluate the profile and long-term prognosis of critically ill patients undergoing tight glucose-control. METHODS: Prospective cohort. All patients admitted to the intensive care unit over 1-year were enrolled. We analyzed demographic data, therapeutic intervention, and short- (during the stay) and long-term (2 years after discharge) mortality. The patients were categorized in 2 groups: tight glucose control and non-tight glucose-control, based on the unit staff decision. RESULTS: From the 603 enrolled patients, 102 (16.9%) underwent tight control (glucose <150 mg/dL) while 501 patients (83.1%) non-tight control. Patients in the TGC-group were more severely ill than those in the non-tight control group [APACHE II score (14 ± 3 versus 11 ± 4, P=0.04), SOFA (4.9 ± 3.2 versus 3.5 ± 3.4, P<0.001) and TISS-24h (25.7 ± 6.9 versus 21.1 ± 7.2, P< 0.001)]. The tight control group patients also had worse prognosis: [acute renal failure (51% versus 18.5%, P<0.001), critical illness neuropathy (16.7% versus 5.6%, P<0.001)] and increased mortality (during the ICU-stay [60.7% versus 17.7%, P<0.001] and within 2-years of the discharge [77.5% versus 23.4%; P<0.001]). CONCLUSION: Critically ill patients needing tight glucose control during the unit stay have more severe disease and have worse short and long-term prognosis.
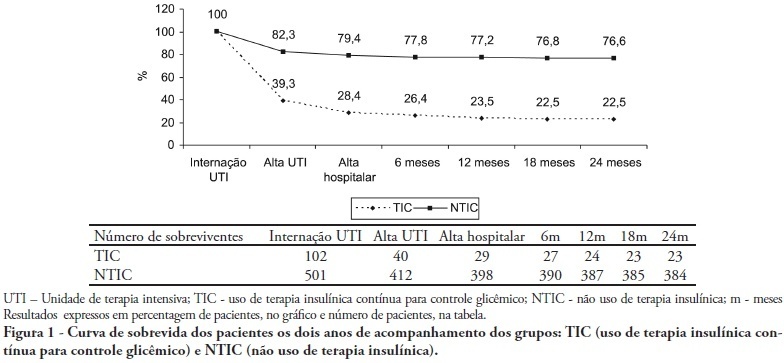
Abstract
Rev Bras Ter Intensiva. 2009;21(3):310-314
DOI 10.1590/S0103-507X2009000300012
Glucose control is a major issue in critical care since landmark publications from the last decade leading to widespread use of strict glucose control in the clinical practice. Subsequent trials showed discordant results that lead to several questions and concerns about benefits and risks of implementing an intensive glucose control protocol. In the midst of all recent controversy, we propose that a new glycemic target -150mg/dl) should be aimed. This target glucose level could offer protection against the deleterious effects of hyperglycemia and at the same time keep patient's safety avoiding hypoglicemia. The article presents a critical review of the current literature on intensive insulin therapy in critically ill patients.
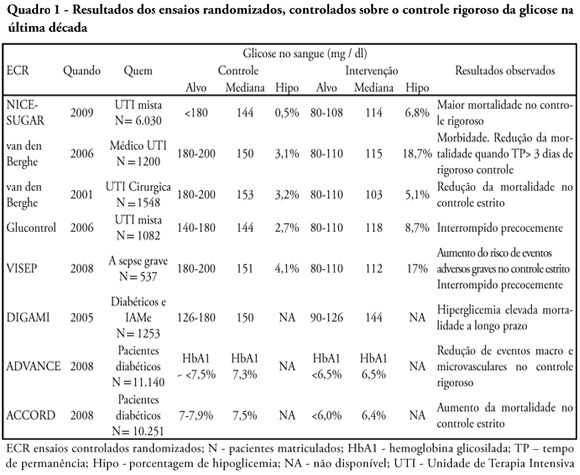
Abstract
Rev Bras Ter Intensiva. 2006;18(3):268-275
DOI 10.1590/S0103-507X2006000300009
BACKGROUND AND OBJECTIVES: Actually tight glycemic control is a major concern in critical care. The objective of this study was to evaluate effectiveness and safety of Yale insulin infusion protocol in a Brazilian medical and surgical intensive care unit. METHODS: Retrospective, before-after cohort study. Selected end-points were mean blood glucose levels, time-to-reach target range of 80 - 140 mg/dL, and percent of blood glucose in target range and hypoglycemia incidence. RESULTS: Were studied 112 patients: 60 in control group (CG) and 52 in protocol group (PG). Bedside blood glucose was measured 5392 times for a mean value of 131.2 ± 14.7 mg/dL in the PG versus 2485 times for a mean value of 181.7 ± 36.1 mg/dL in the CG. Blood glucose values were in the target range 65% and 32% of the times, respectively for PG and CG groups (p < 0.001). The median time to reach glucose target range was 7 h (range 4 -10 h) for PG and 96 hr (range 46 - 278 h) for CG (p < 0.001). Incidence of severe hypoglycemia did not reach difference statistically significant: 4 patients in PG versus 2 patients in CG. CONCLUSIONS: Yale insulin infusion protocol was effective and safe to improve blood glucose control in a Brazilian medical and surgical intensive care unit.
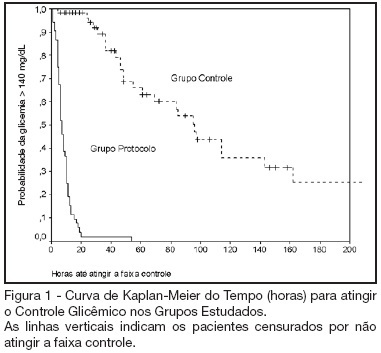
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)