Abstract
Crit Care Sci. 2023;35(2):217-225
DOI 10.5935/2965-2774.20230289-pt
To analyze the effect of CytoSorb® on mortality, interleukin levels, vasopressor use and adverse events in patients with sepsis.
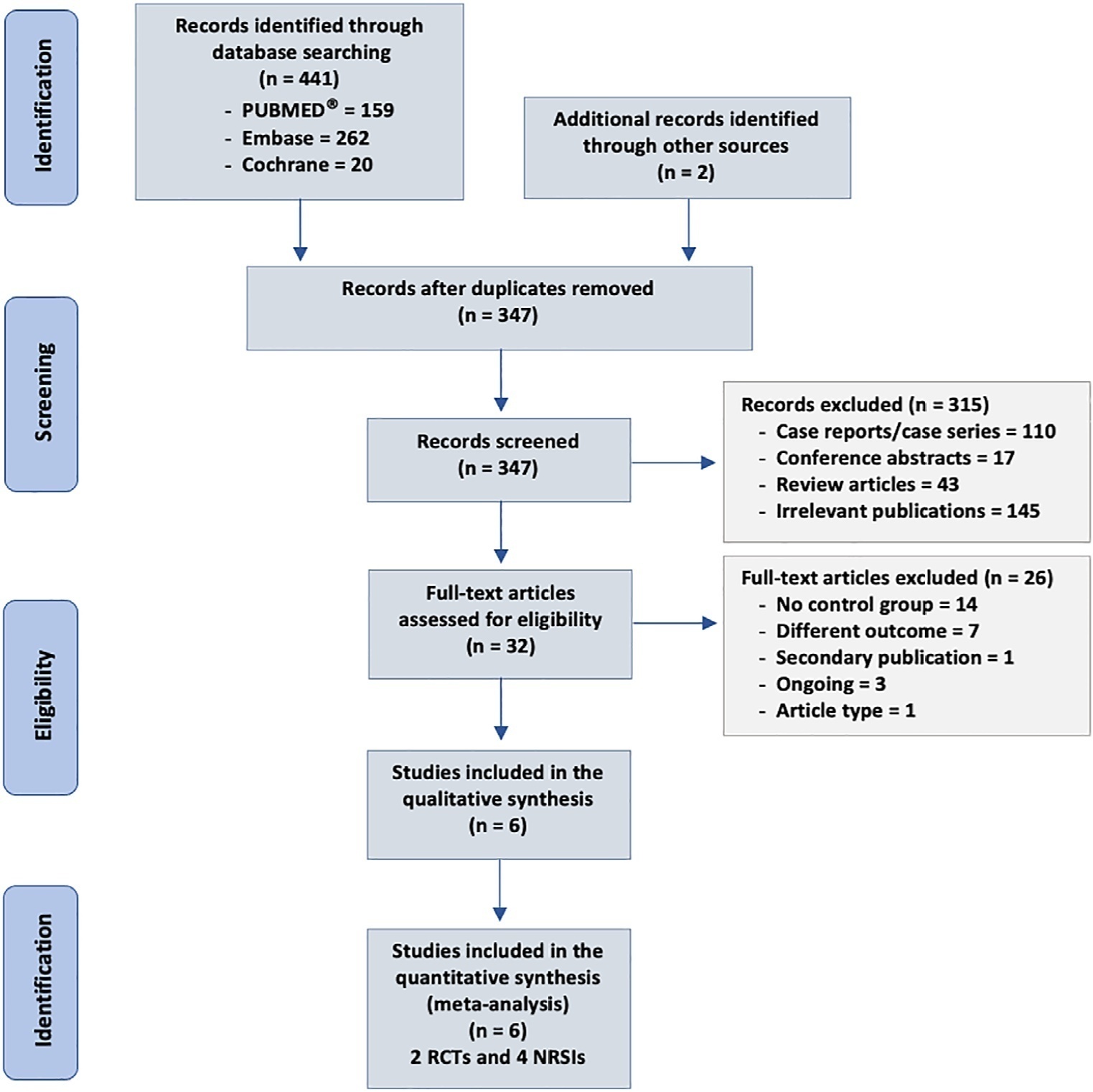
We searched MEDLINE®, Embase and the Cochrane Library for randomized controlled trials and cohort studies that reported the use of CytoSorb® among septic patients. The primary outcome was mortality, and secondary outcomes included the use of vasopressors, levels of inflammatory markers, predicted versus observed mortality, length of stay in the intensive care unit, and adverse events.
We included 6 studies enrolling 413 patients, and assessment for risk of bias indicated variations in study quality from high to moderate. The overall mortality rate was 45%, and no significant effect on mortality was found at 28 - 30 days (RR 0.98 [0.12 - 8.25] for the randomized clinical trial and RR 0.74 [0.49 - 1.13] for cohort studies). We did not perform a metanalysis for other outcomes due to the small number of studies found or the lack of data.
Our study found very low certainty evidence, due to imprecision, risk of bias, and heterogeneity, thereby showing no benefit of CytoSorb® use in terms of mortality at 28 - 30 days. We cannot recommend the use of CytoSorb® in septic or septic shock patients outside clinical trials. Further high-quality randomized trials with a common intervention arm are needed to evaluate the influence of CytoSorb® in this population.
Abstract
Rev Bras Ter Intensiva. 2022;34(1):96-106
DOI 10.5935/0103-507X.20220004-en
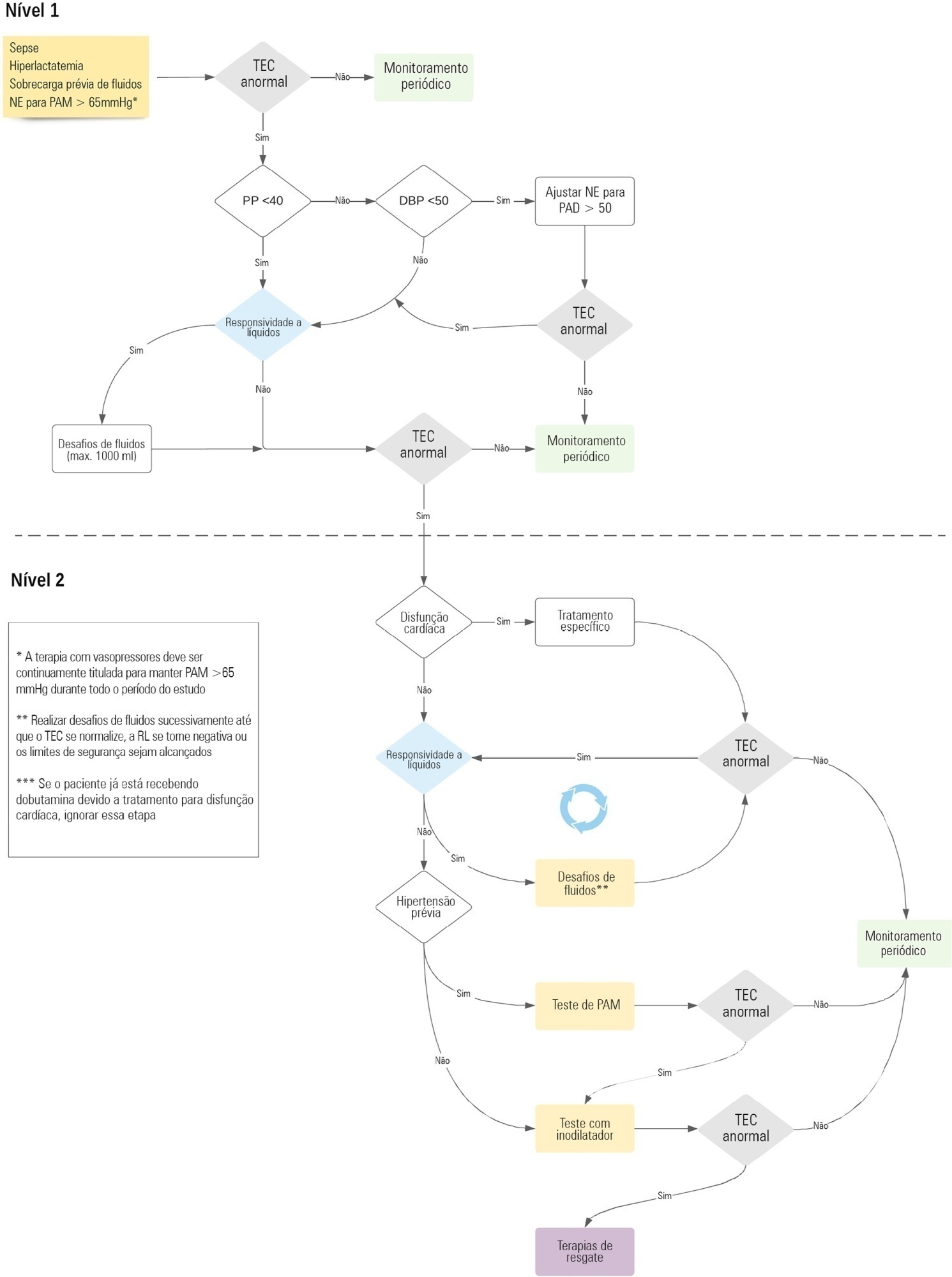
Early reversion of sepsis-induced tissue hypoperfusion is essential for survival in septic shock. However, consensus regarding the best initial resuscitation strategy is lacking given that interventions designed for the entire population with septic shock might produce unnecessary fluid administration. This article reports the rationale, study design and analysis plan of the ANDROMEDA-2 study, which aims to determine whether a peripheral perfusion-guided strategy consisting of capillary refill time-targeted resuscitation based on clinical and hemodynamic phenotypes is associated with a decrease in a composite outcome of mortality, time to organ support cessation, and hospital length of stay compared to standard care in patients with early (< 4 hours of diagnosis) septic shock.
The ANDROMEDA-2 study is a multicenter, multinational randomized controlled trial. In the intervention group, capillary refill time will be measured hourly for 6 hours. If abnormal, patients will enter an algorithm starting with pulse pressure assessment. Patients with pulse pressure less than 40mmHg will be tested for fluid responsiveness and receive fluids accordingly. In patients with pulse pressure > 40mmHg, norepinephrine will be titrated to maintain diastolic arterial pressure > 50mmHg. Patients who fail to normalize capillary refill time after the previous steps will be subjected to critical care echocardiography for cardiac dysfunction evaluation and subsequent management. Finally, vasopressor and inodilator tests will be performed to further optimize perfusion. A sample size of 1,500 patients will provide 88% power to demonstrate superiority of the capillary refill time-targeted strategy.
If hemodynamic phenotype-based, capillary refill time-targeted resuscitation demonstrates to be a superior strategy, care processes in septic shock resuscitation can be optimized with bedside tools.
Abstract
Rev Bras Ter Intensiva. 2021;33(2):231-242
DOI 10.5935/0103-507X.20210030
To report the prevalence and outcomes of sepsis in children admitted to public and private hospitals.
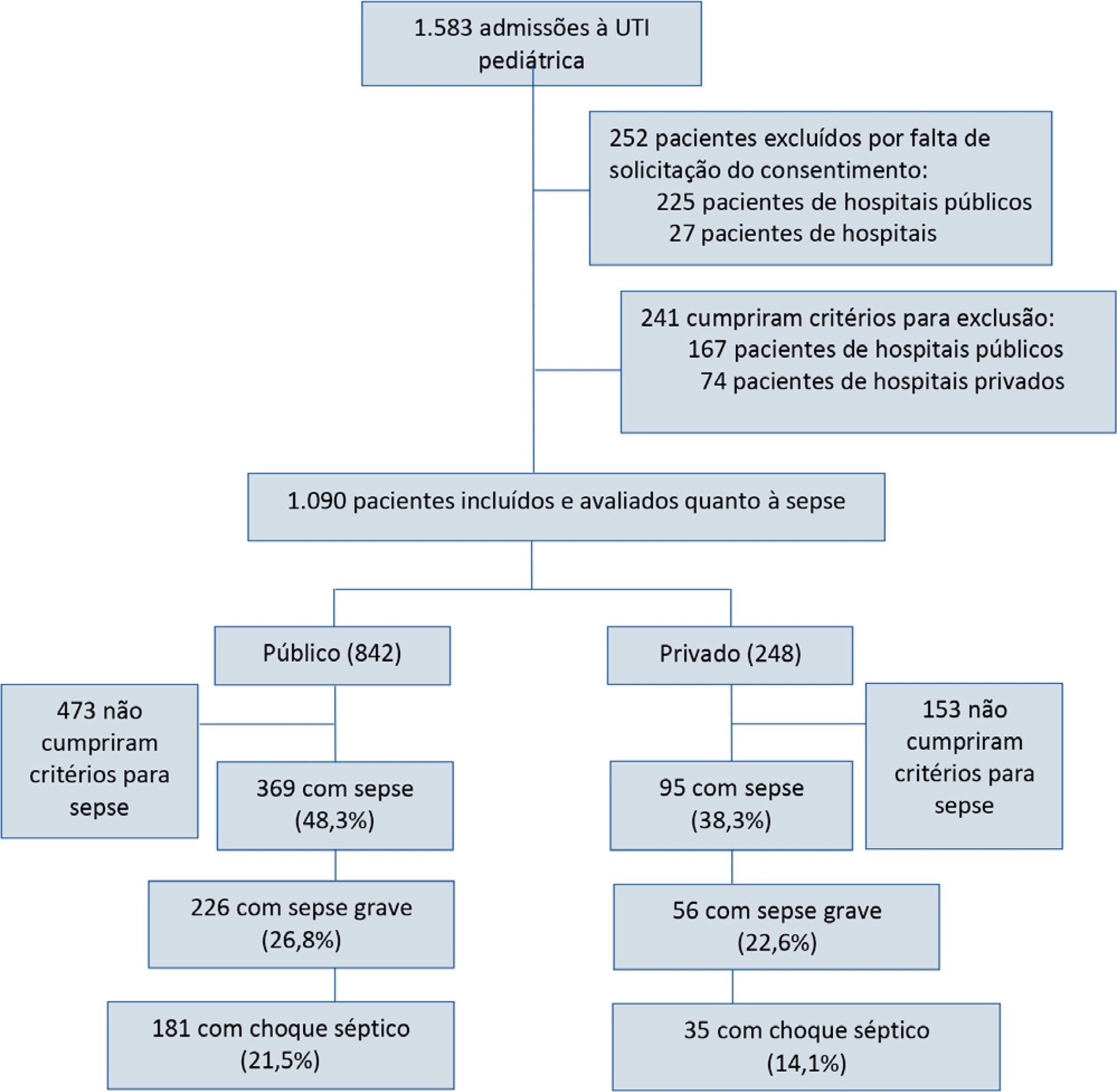
Post hoc analysis of the Latin American Pediatric Sepsis Study (LAPSES) data, a cohort study that analyzed the prevalence and outcomes of sepsis in critically ill children with sepsis on admission at 21 pediatric intensive care units in five Latin American countries.
Of the 464 sepsis patients, 369 (79.5%) were admitted to public hospitals and 95 (20.5%) to private hospitals. Compared to those admitted to private hospitals, sepsis patients admitted to public hospitals did not differ in age, sex, immunization status, hospital length of stay or type of admission but had higher rates of septic shock, higher Pediatric Risk of Mortality (PRISM), Pediatric Index of Mortality 2 (PIM 2), and Pediatric Logistic Organ Dysfunction (PELOD) scores, and higher rates of underlying diseases and maternal illiteracy. The proportion of patients admitted from pediatric wards and sepsis-related mortality were higher in public hospitals. Multivariate analysis did not show any correlation between mortality and the type of hospital, but mortality was associated with greater severity on pediatric intensive care unit admission in patients from public hospitals.
In this sample of critically ill children from five countries in Latin America, the prevalence of septic shock within the first 24 hours at admission and sepsis-related mortality were higher in public hospitals than in private hospitals. Higher sepsis-related mortality in children admitted to public pediatric intensive care units was associated with greater severity on pediatric intensive care unit admission but not with the type of hospital. New studies will be necessary to elucidate the causes of the higher prevalence and mortality of pediatric sepsis in public hospitals.
Abstract
Rev Bras Ter Intensiva. 2020;32(2):245-250
DOI 10.5935/0103-507X.20200029
To assess the relationship between time to focus clearance and hospital mortality in patients with sepsis and septic shock.
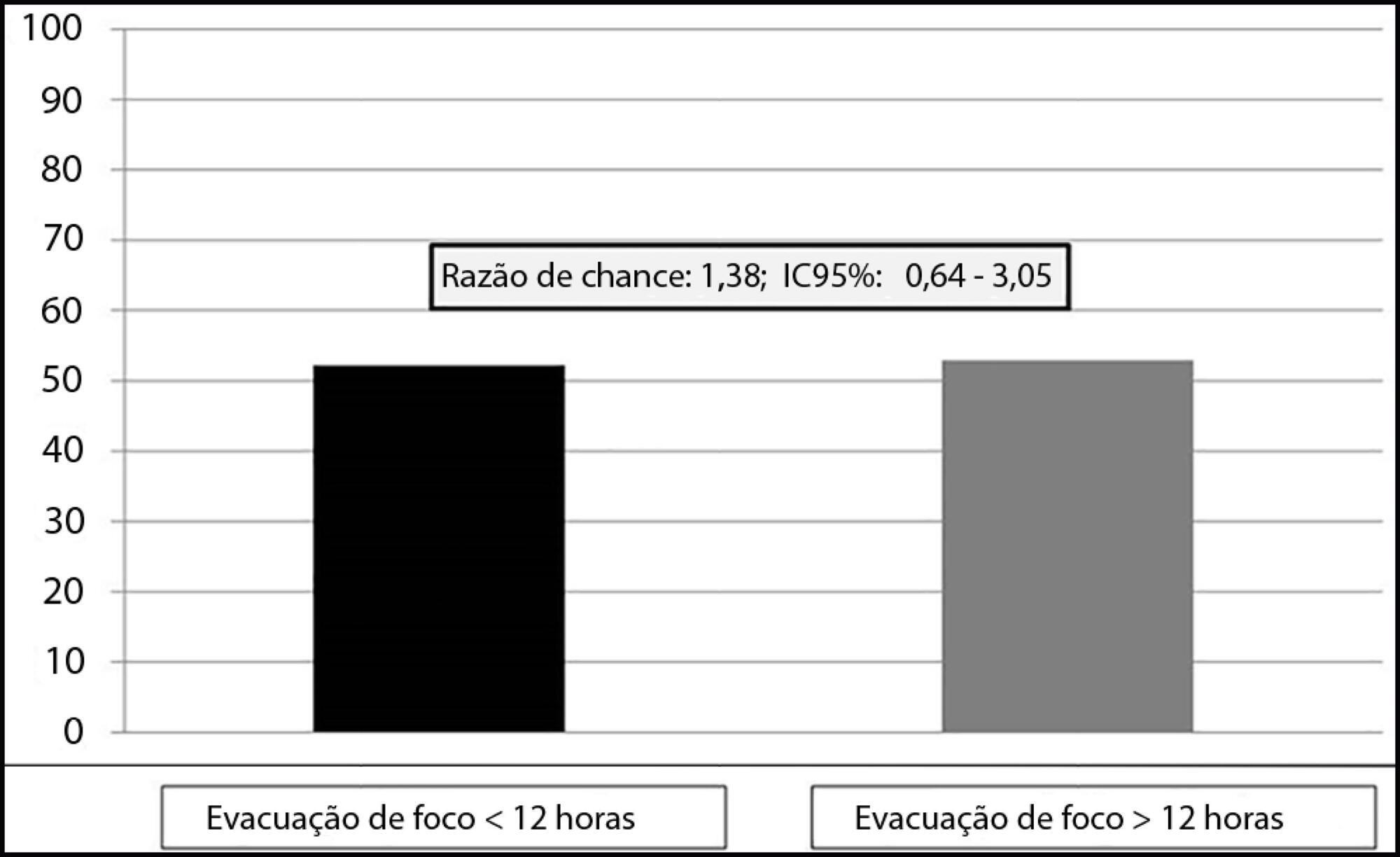
This was an observational, single-center study with a retrospective analysis of the time to clearance of abdominal septic focus. Patients were classified according to the time to focus clearance into an early (≤ 12 hours) or delayed (> 12 hours) group.
A total of 135 patients were evaluated. There was no association between time to focus clearance and hospital mortality (≤ 12 hours versus > 12 hours): 52.3% versus 52.9%, with p = 0.137.
There was no difference in hospital mortality among patients with sepsis or septic shock who had an infectious focus evacuated before or after 12 hours after the diagnosis of sepsis.
Abstract
Rev Bras Ter Intensiva. 2018;30(4):443-452
DOI 10.5935/0103-507X.20180064
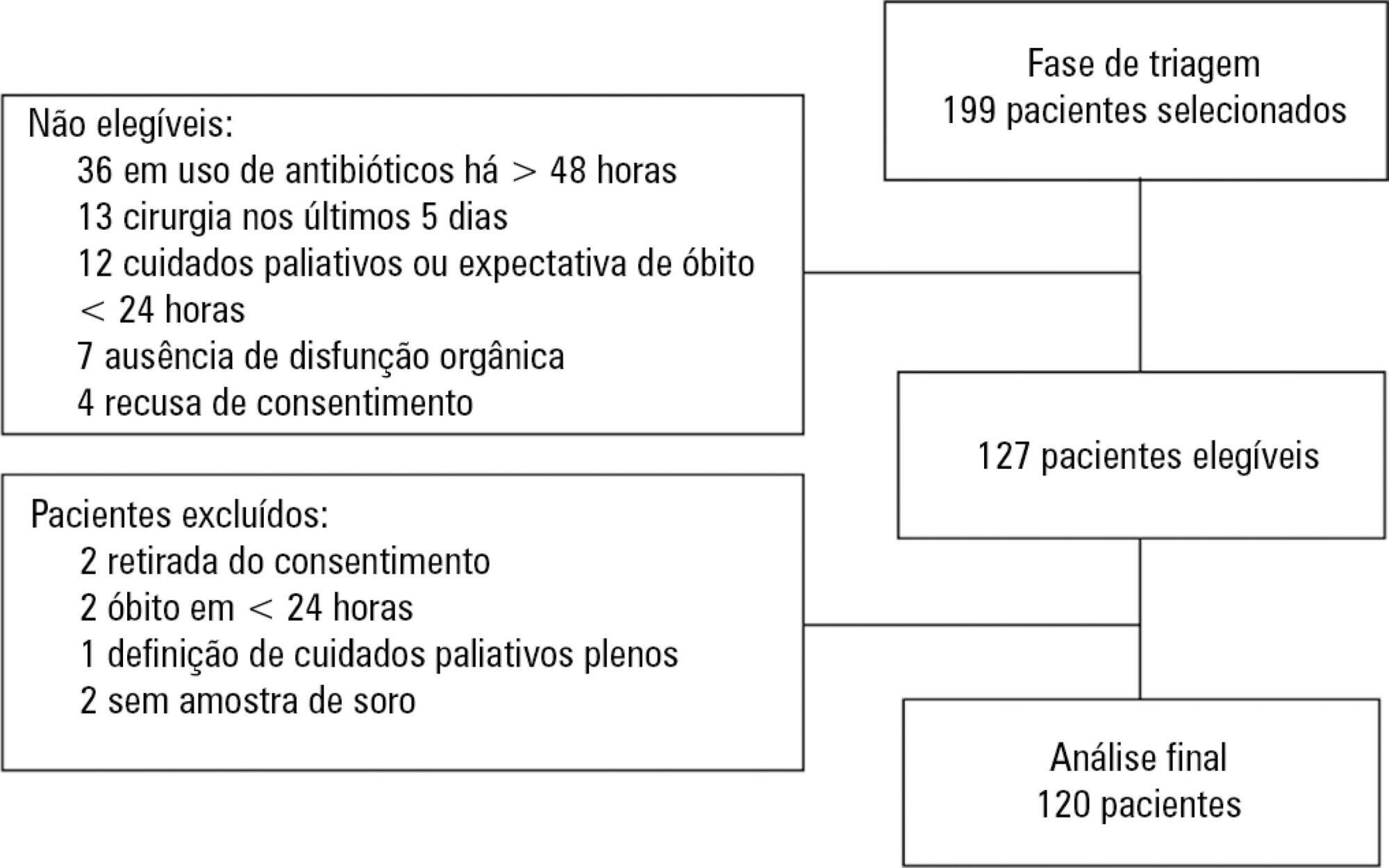
To evaluate the accuracy of IL-3 to predict the outcome of septic patients.
Prospective cohort study with adult patients in an intensive care unit with sepsis or septic shock diagnosed within the previous 48 hours. Circulating IL-3 levels were measured upon inclusion (day 1) and on days 3 and 7. The primary outcome was hospital mortality.
One hundred and twenty patients were included. Serum levels of IL-3 on day 1 were significantly higher among patients who died than among patients who survived the hospital stay (91.2pg/mL versus 36pg/mL, p = 0.024). In a Cox survival model considering the IL-3 levels at inclusion, age and sequential SOFA, IL-3 values remained independently associated with mortality (HR 1.032; 95%CI 1.010 - 1.055; p = 0.005). An receiver operating characteristic curve was built to further investigate the accuracy of IL-3, with an area under the curve of 0.62 (95%CI 0.51 - 0.73; p = 0.024) for hospital mortality. A cutoff initial IL-3 value above 127.5pg/mL was associated with hospital mortality (OR 2.97; 95%CI: 1.27 - 6.97; p = 0.0019) but with a low performance (82% for specificity, 39% for sensibility, 53% for the positive predictive value, 72% for the negative predictive value, 0.73 for the negative likelihood and 2.16 for the positive likelihood ratio).
Higher levels of IL-3 are shown to be independently associated with hospital mortality in septic patients but with poor clinical performance.
Abstract
Rev Bras Ter Intensiva. 2019;31(1):47-56
DOI 10.5935/0103-507X.20190011
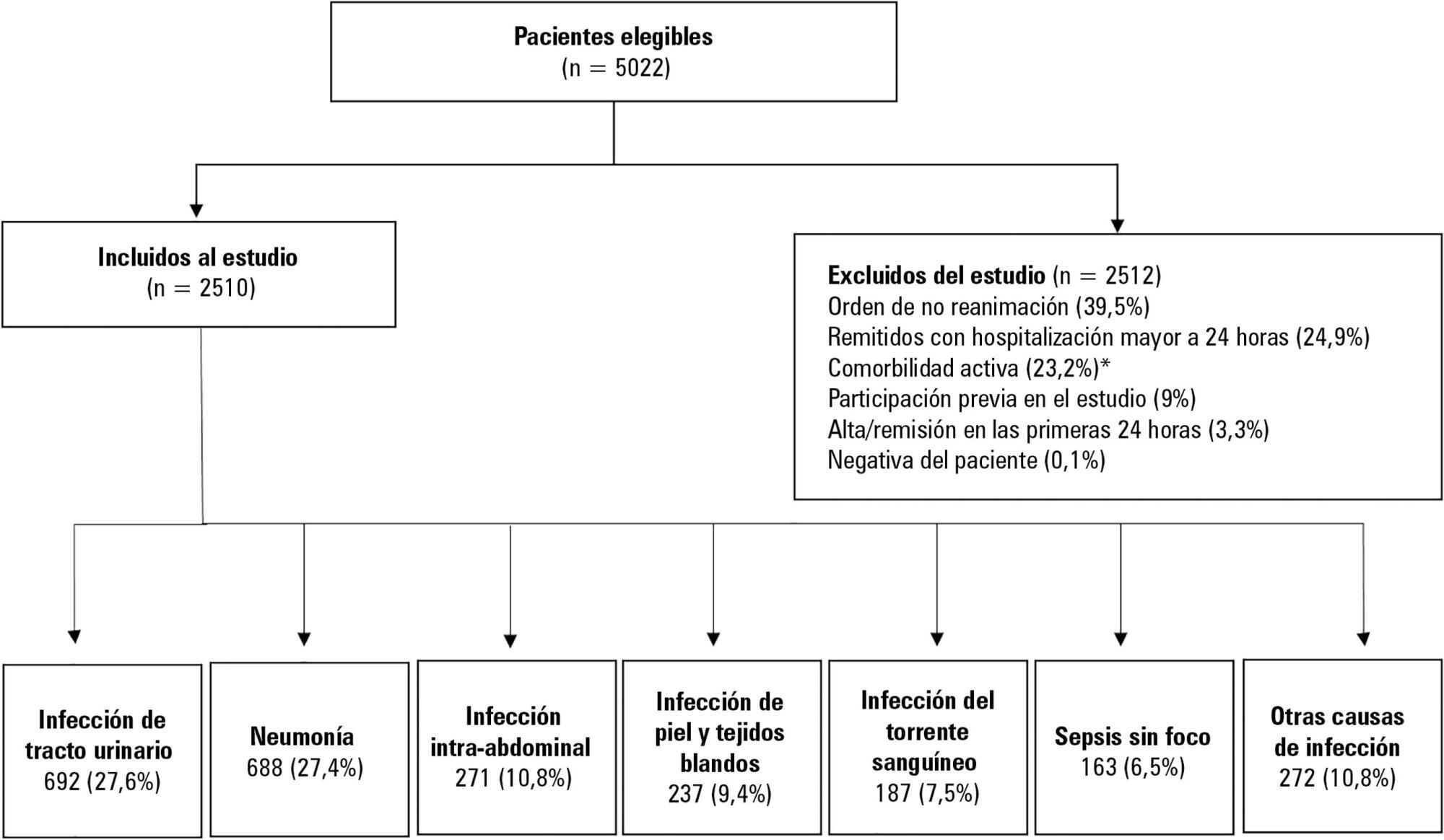
To determine the association between the primary site of infection and in-hospital mortality as the main outcome, or the need for admission to the intensive care unit as a secondary outcome, in patients with sepsis admitted to the emergency department.
This was a secondary analysis of a multicenter prospective cohort. Patients included in the study were older than 18 years with a diagnosis of severe sepsis or septic shock who were admitted to the emergency departments of three tertiary care hospitals. Of the 5022 eligible participants, 2510 were included. Multiple logistic regression analysis was performed for mortality.
The most common site of infection was the urinary tract, present in 27.8% of the cases, followed by pneumonia (27.5%) and intra-abdominal focus (10.8%). In 5.4% of the cases, no definite site of infection was identified on admission. Logistic regression revealed a significant association between the following sites of infection and in-hospital mortality when using the urinary infection group as a reference: pneumonia (OR 3.4; 95%CI, 2.2 - 5.2; p < 0.001), skin and soft tissues (OR 2.6; 95%CI, 1.4 - 5.0; p = 0.003), bloodstream (OR 2.0; 95%CI, 1.1 - 3.6; p = 0.018), without specific focus (OR 2.0; 95%CI, 1.1 - 3.8; p = 0.028), and intra-abdominal focus (OR 1.9; 95%CI, 1.1 - 3.3; p = 0.024).
There is a significant association between the different sites of infection and in-hospital mortality or the need for admission to an intensive care unit in patients with sepsis or septic shock. Urinary tract infection shows the lowest risk, which should be considered in prognostic models of these conditions.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)