Abstract
Crit Care Sci. 2024;36:e20240196en
DOI 10.62675/2965-2774.20240196-en
To provide insights into the potential benefits of goal-directed therapy guided by FloTrac in reducing postoperative complications and improving outcomes.
We performed a systematic review and meta-analysis of randomized controlled trials to evaluate goal-directed therapy guided by FloTrac in major surgery, comparing goal-directed therapy with usual care or invasive monitoring in cardiac and noncardiac surgery subgroups. The quality of the articles and evidence were evaluated with a risk of bias tool and GRADE.
We included 29 randomized controlled trials with 3,468 patients. Goal-directed therapy significantly reduced the duration of hospital stay (mean difference -1.43 days; 95%CI 2.07 to -0.79; I2 81%), intensive care unit stay (mean difference -0.77 days; 95%CI -1.18 to -0.36; I2 93%), and mechanical ventilation (mean difference -2.48 hours, 95%CI -4.10 to -0.86, I2 63%). There was no statistically significant difference in mortality, myocardial infarction, acute kidney injury or hypotension, but goal-directed therapy significantly reduced the risk of heart failure or pulmonary edema (RR 0.46; 95%CI 0.23 - 0.92; I2 0%).
Goal-directed therapy guided by the FloTrac sensor improved clinical outcomes and shortened the length of stay in the hospital and intensive care unit in patients undergoing major surgery. Further research can validate these results using specific protocols and better understand the potential benefits of FloTrac beyond these outcomes.
Abstract
Rev Bras Ter Intensiva. 2022;34(1):87-95
DOI 10.5935/0103-507x.20220003-en
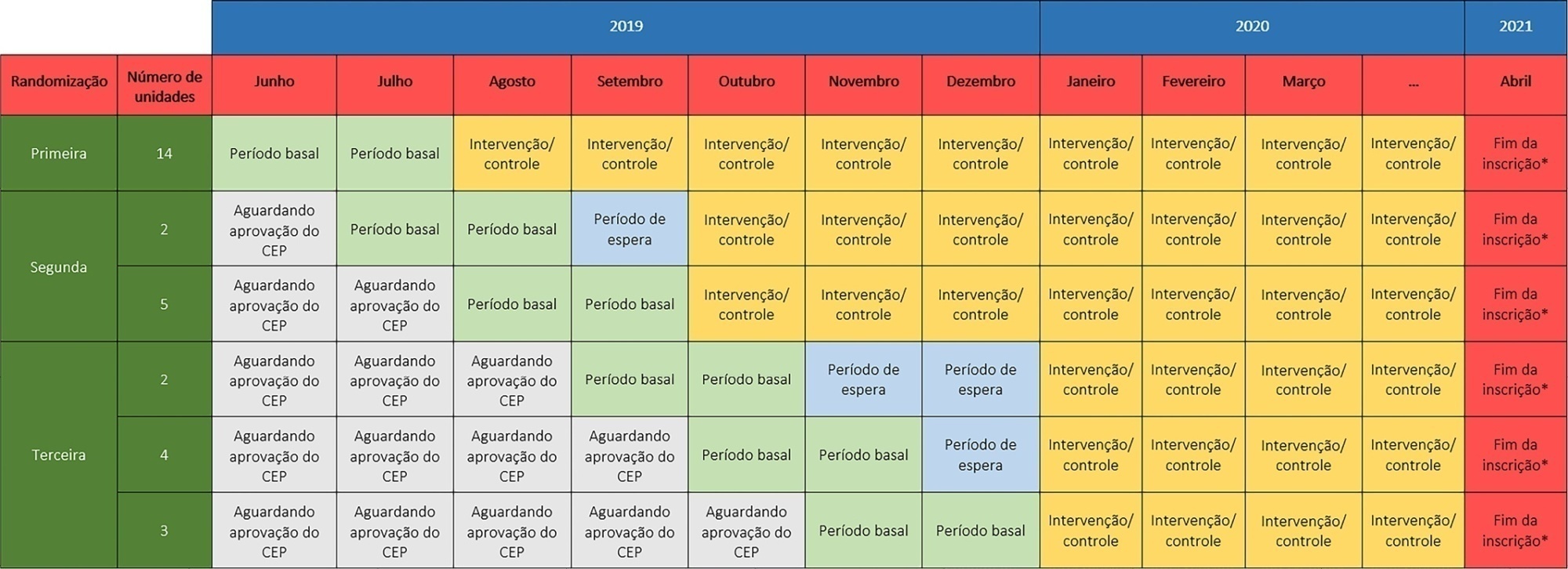
The TELE-critical Care verSus usual Care On ICU PErformance (TELESCOPE) trial aims to assess whether a complex telemedicine intervention in intensive care units, which focuses on daily multidisciplinary rounds performed by remote intensivists, will reduce intensive care unit length of stay compared to usual care.
The TELESCOPE trial is a national, multicenter, controlled, open label, cluster randomized trial. The study tests the effectiveness of daily multidisciplinary rounds conducted by an intensivist through telemedicine in Brazilian intensive care units. The protocol was approved by the local Research Ethics Committee of the coordinating study center and by the local Research Ethics Committee from each of the 30 intensive care units, following Brazilian legislation. The trial is registered with ClinicalTrials. gov (NCT03920501). The primary outcome is intensive care unit length of stay, which will be analyzed accounting for the baseline period and cluster structure of the data and adjusted by prespecified covariates. Secondary exploratory outcomes included intensive care unit performance classification, in-hospital mortality, incidence of nosocomial infections, ventilator-free days at 28 days, rate of patients receiving oral or enteral feeding, rate of patients under light sedation or alert and calm, and rate of patients under normoxemia.
According to the trial’s best practice, we report our statistical analysis prior to locking the database and beginning analyses. We anticipate that this reporting practice will prevent analysis bias and improve the interpretation of the reported results.
Abstract
Rev Bras Ter Intensiva. 2022;34(1):96-106
DOI 10.5935/0103-507X.20220004-en
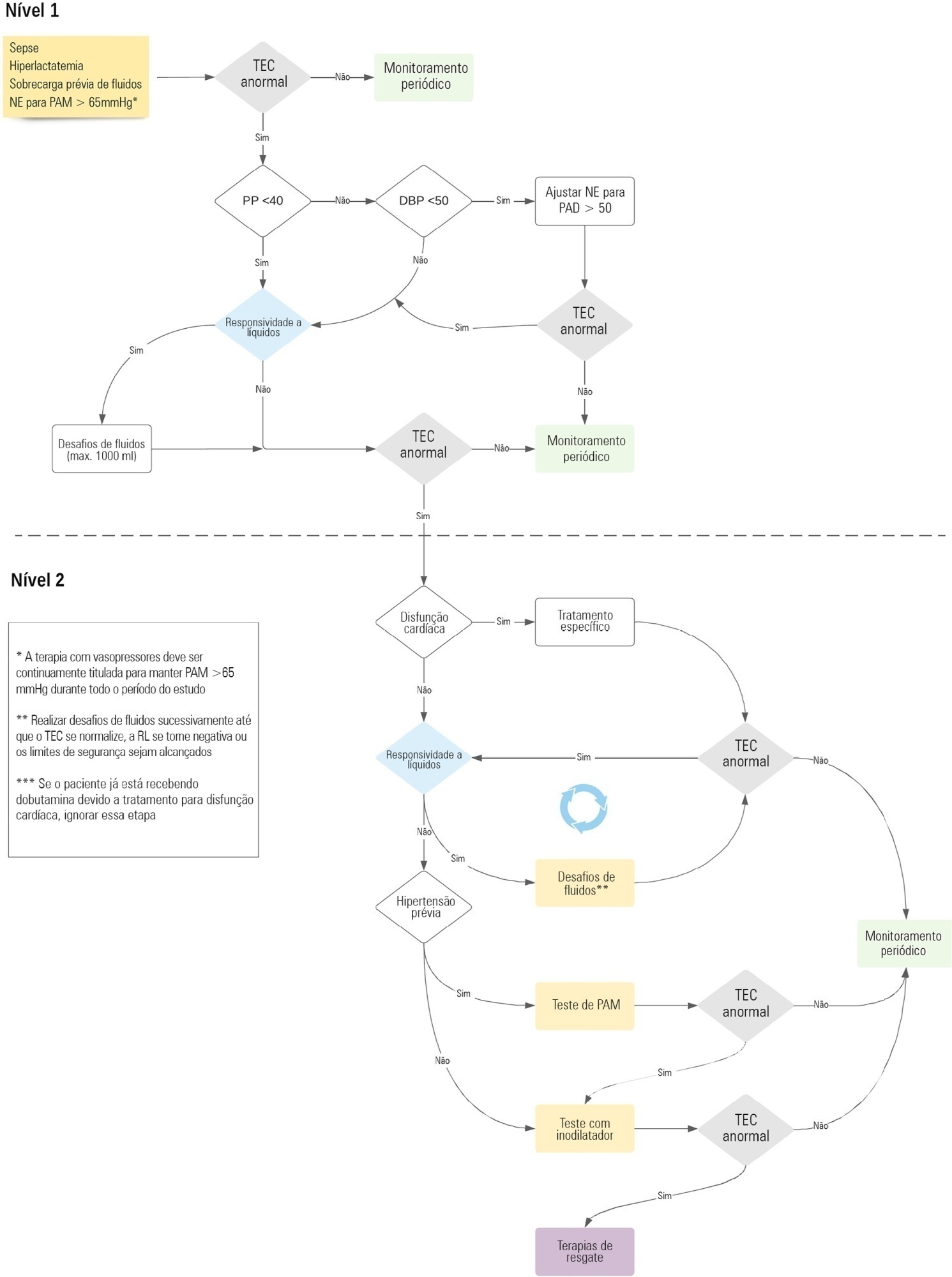
Early reversion of sepsis-induced tissue hypoperfusion is essential for survival in septic shock. However, consensus regarding the best initial resuscitation strategy is lacking given that interventions designed for the entire population with septic shock might produce unnecessary fluid administration. This article reports the rationale, study design and analysis plan of the ANDROMEDA-2 study, which aims to determine whether a peripheral perfusion-guided strategy consisting of capillary refill time-targeted resuscitation based on clinical and hemodynamic phenotypes is associated with a decrease in a composite outcome of mortality, time to organ support cessation, and hospital length of stay compared to standard care in patients with early (< 4 hours of diagnosis) septic shock.
The ANDROMEDA-2 study is a multicenter, multinational randomized controlled trial. In the intervention group, capillary refill time will be measured hourly for 6 hours. If abnormal, patients will enter an algorithm starting with pulse pressure assessment. Patients with pulse pressure less than 40mmHg will be tested for fluid responsiveness and receive fluids accordingly. In patients with pulse pressure > 40mmHg, norepinephrine will be titrated to maintain diastolic arterial pressure > 50mmHg. Patients who fail to normalize capillary refill time after the previous steps will be subjected to critical care echocardiography for cardiac dysfunction evaluation and subsequent management. Finally, vasopressor and inodilator tests will be performed to further optimize perfusion. A sample size of 1,500 patients will provide 88% power to demonstrate superiority of the capillary refill time-targeted strategy.
If hemodynamic phenotype-based, capillary refill time-targeted resuscitation demonstrates to be a superior strategy, care processes in septic shock resuscitation can be optimized with bedside tools.
Abstract
Rev Bras Ter Intensiva. 2021;33(1):125-137
DOI 10.5935/0103-507X.20210014
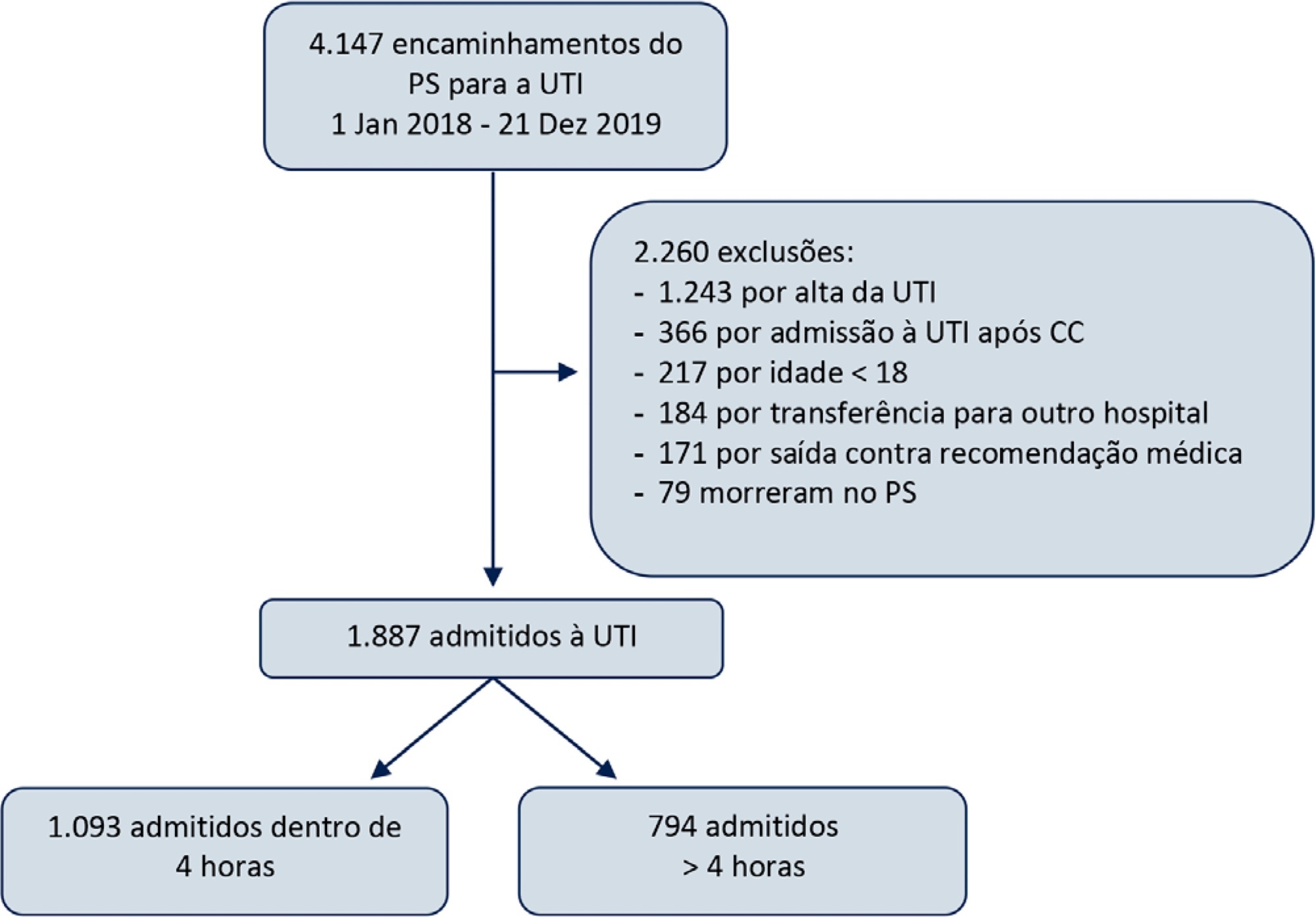
To study the impact of delayed admission by more than 4 hours on the outcomes of critically ill patients.
This was a retrospective observational study in which adult patients admitted directly from the emergency department to the intensive care unit were divided into two groups: Timely Admission if they were admitted within 4 hours and Delayed Admission if admission was delayed for more than 4 hours. Intensive care unit length of stay and hospital/intensive care unit mortality were compared between the groups. Propensity score matching was performed to correct for imbalances. Logistic regression analysis was used to explore delayed admission as an independent risk factor for intensive care unit mortality.
During the study period, 1,887 patients were admitted directly from the emergency department to the intensive care unit, with 42% being delayed admissions. Delayed patients had significantly longer intensive care unit lengths of stay and higher intensive care unit and hospital mortality. These results were persistent after propensity score matching of the groups. Delayed admission was an independent risk factor for intensive care unit mortality (OR = 2.6; 95%CI 1.9 - 3.5; p < 0.001). The association of delay and intensive care unit mortality emerged after a delay of 2 hours and was highest after a delay of 4 hours.
Delayed admission to the intensive care unit from the emergency department is an independent risk factor for intensive care unit mortality, with the strongest association being after a delay of 4 hours.
Abstract
Rev Bras Ter Intensiva. 2020;32(2):301-307
DOI 10.5935/0103-507X.20200047
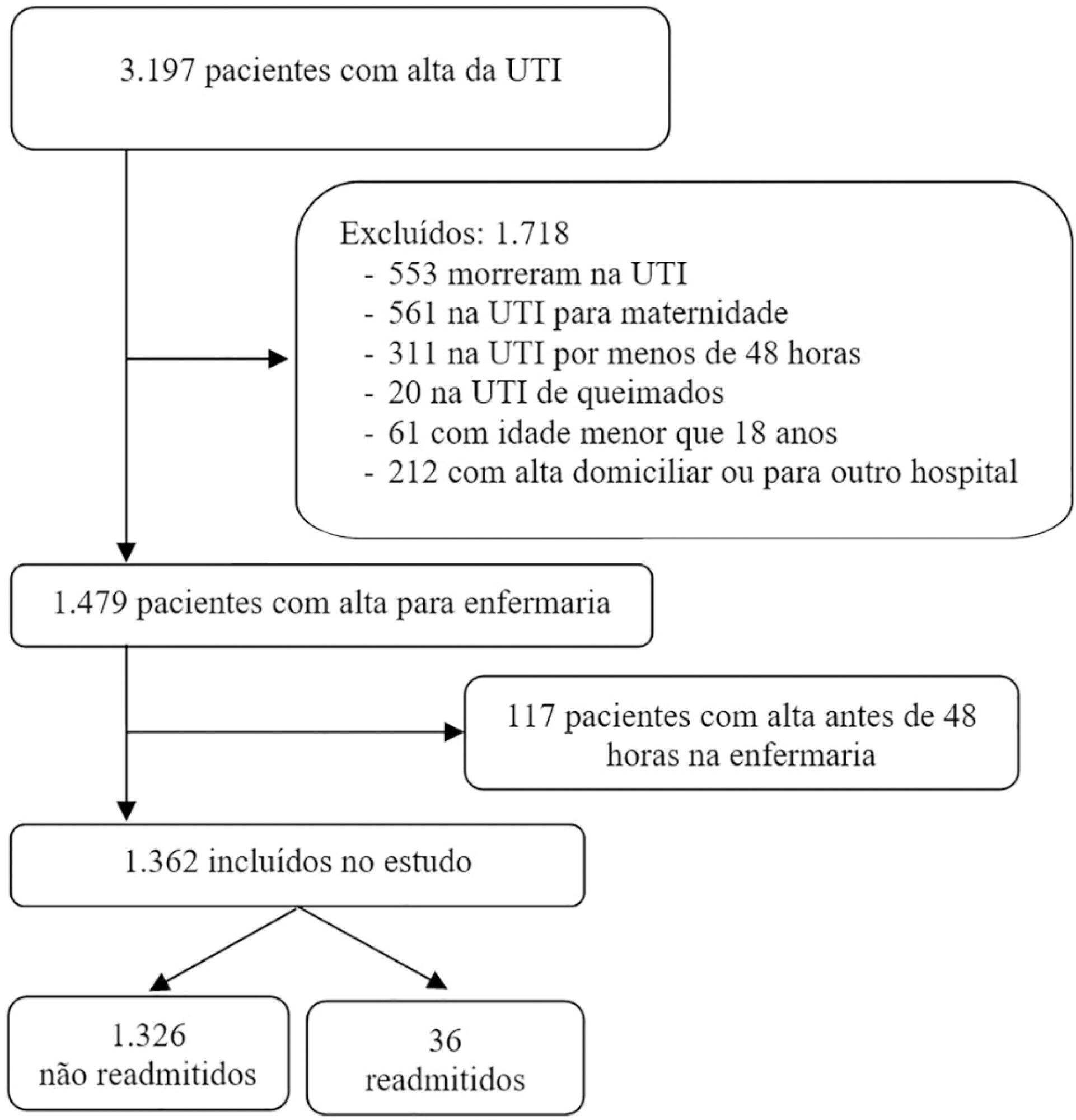
To evaluate the hypothesis that the Modified Early Warning Score (MEWS) at the time of intensive care unit discharge is associated with readmission and to identify the MEWS that most reliably predicts intensive care unit readmission within 48 hours of discharge.
This was a retrospective observational study of the MEWSs of discharged patients from the intensive care unit. We compared the demographics, severity scores, critical illness characteristics, and MEWSs of readmitted and non-readmitted patients, identified factors associated with readmission in a logistic regression model, constructed a Receiver Operating Characteristic (ROC) curve of the MEWS in predicting the probability of readmission, and presented the optimum criterion with the highest sensitivity and specificity.
The readmission rate was 2.6%, and the MEWS was a significant predictor of readmission, along with intensive care unit length of stay > 10 days and tracheostomy. The ROC curve of the MEWS in predicting the readmission probability had an AUC of 0.82, and a MEWS > 6 carried a sensitivity of 0.78 (95%CI 0.66 - 0.9) and specificity of 0.9 (95%CI 0.87 - 0.93).
The MEWS is associated with intensive care unit readmission, and a score > 6 has excellent accuracy as a prognostic predictor.
Abstract
Rev Bras Ter Intensiva. 2020;32(1):92-98
DOI 10.5935/0103-507X.20200014
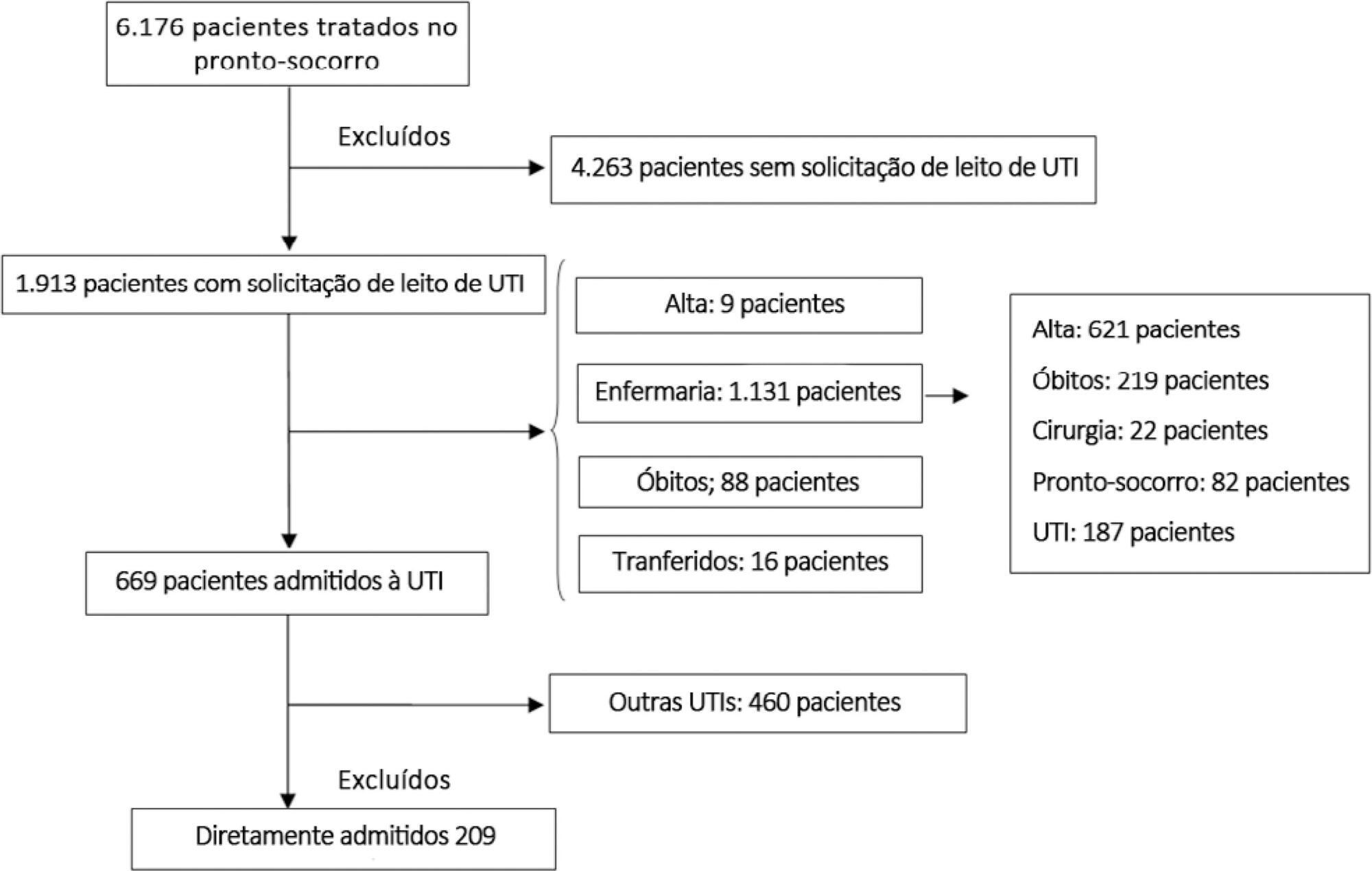
To examine the impact of delayed transfer from the emergency room into the intensive care unit on the length of intensive care unit stay and death.
This prospective, cohort study performed in a tertiary academic hospital obtained data from 1913 patients admitted to the emergency room with a documented request for admission into the intensive care unit. The patients admitted directly into the medical-surgical intensive care unit (n = 209) were categorized into tertiles according to their waiting time for intensive care unit admission (Group 1: < 637 min, Group 2: 637 to 1602 min, and Group 3: > 1602 min). Patients who stayed in the intensive care unit for longer than 3.2 days (median time of intensive care unit length of stay of all patients) were considered as having a prolonged intensive care unit stay.
A total of 6,176 patients were treated in the emergency room during the study period, among whom 1,913 (31%) required a bed in the intensive care unit. The median length of stay in the emergency room was 17 hours [9 to 33 hours]. Hospitalization for infection/sepsis was an independent predictor of prolonged intensive care unit stay (OR 2.75 95%CI 1.38 - 5.48, p = 0.004), but waiting time for intensive care unit admission was not. The mortality rate was higher in Group 3 (38%) than in Group 1 (31%) but the difference was not statistically significant.
Delayed admission into the intensive care unit from the emergency room did not result in an increased intensive care unit stay or mortality.
Abstract
Rev Bras Ter Intensiva. 2019;31(3):361-367
DOI 10.5935/0103-507X.20190059
To compare the impact of two fast-track strategies regarding the extubation time and removal of invasive mechanical ventilation in adults after cardiac surgery on clinical and hospital outcomes.
This was a retrospective cohort study with patients undergoing cardiac surgery. Patients were classified according to the extubation time as the Control Group (extubated 6 hours after admission to the intensive care unit, with a maximum mechanical ventilation time of 18 hours), Group 1 (extubated in the operating room after surgery) and Group 2 (extubated within 6 hours after admission to the intensive care unit). The primary outcomes analyzed were vital capacity on the first postoperative day, length of hospital stay, and length of stay in the intensive care unit. The secondary outcomes were reintubation, hospital-acquired pneumonia, sepsis, and death.
For the 223 patients evaluated, the vital capacity was lower in Groups 1 and 2 compared to the Control (p = 0.000 and p = 0.046, respectively). The length of stay in the intensive care unit was significantly lower in Groups 1 and 2 compared to the Control (p = 0.009 and p = 0.000, respectively), whereas the length of hospital stay was lower in Group 1 compared to the Control (p = 0.014). There was an association between extubation in the operating room (Group 1) with reintubation (p = 0.025) and postoperative complications (p = 0.038).
Patients undergoing fast-track management with extubation within 6 hours had shorter stays in the intensive care unit without increasing postoperative complications and death. Patients extubated in the operating room had a shorter hospital stay and a shorter stay in the intensive care unit but showed an increase in the frequency of reintubation and postoperative complications.
Abstract
Rev Bras Ter Intensiva. 2018;30(4):496-507
DOI 10.5935/0103-507X.20180071
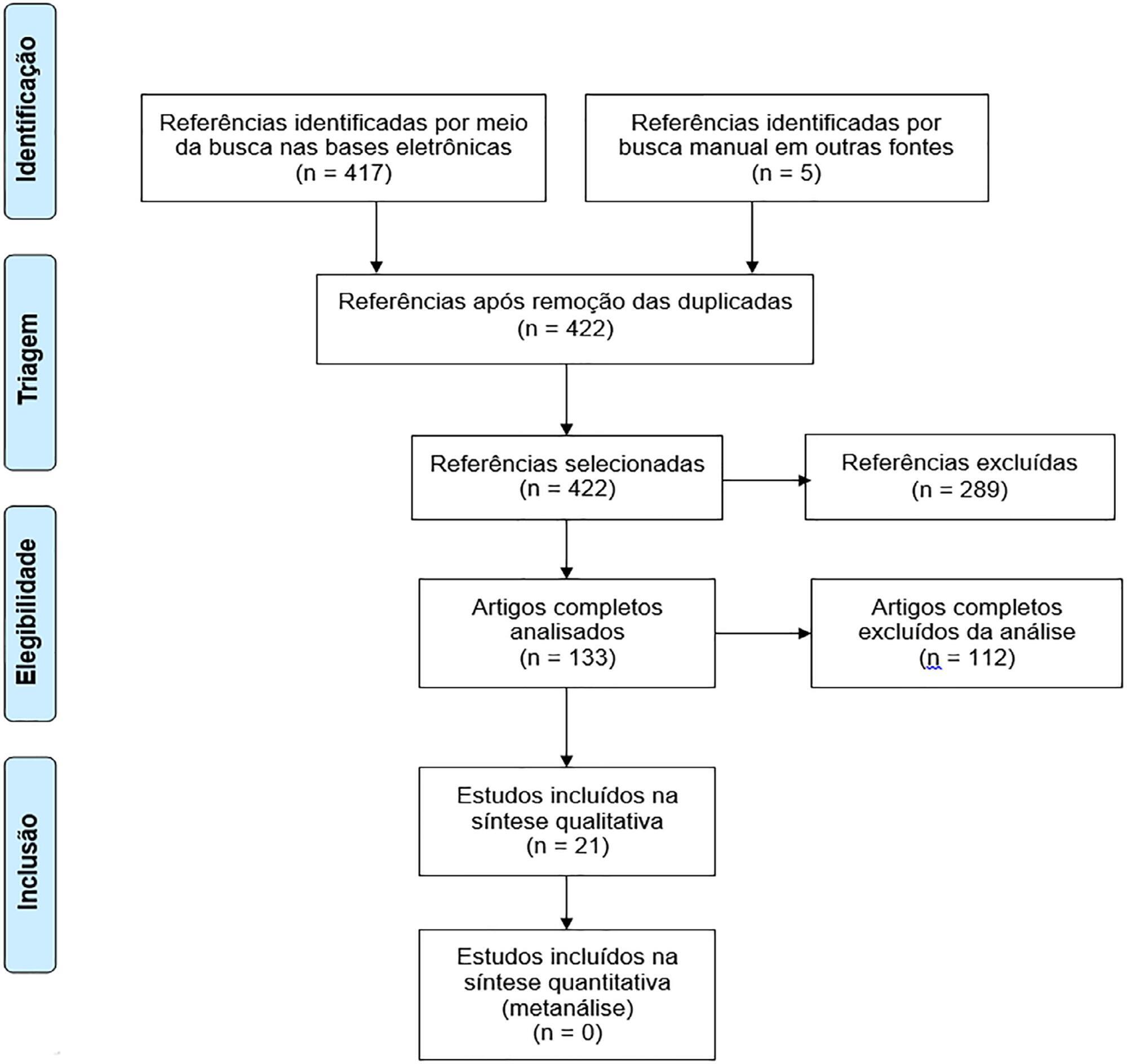
To assess the long-term, health-related quality of life of intensive care unit survivors by systematic review.
The search for, and selection and analysis of, observational studies that assessed the health-related quality of life of intensive care unit survivors in the electronic databases LILACS and MEDLINE® (accessed through PubMed) was performed using the indexed MESH terms "quality of life [MeSH Terms]" AND "critically illness [MeSH Terms]". Studies on adult patients without specific prior diseases published in English in the last 5 years were included in this systematic review. The citations were independently selected by three reviewers. Data were standardly and independently retrieved by two reviewers, and the quality of the studies was assessed using the Newcastle-Ottawa scale.
In total, 19 observational cohort and 2 case-control studies of 57,712 critically ill patients were included. The follow-up time of the studies ranged from 6 months to 6 years, and most studies had a 6-month or 1-year follow up. The health-related quality of life was assessed using two generic tools, the EuroQol and the Short Form Health Survey. The overall quality of the studies was low.
Long-term, health-related quality of life is compromised among intensive care unit survivors compared with the corresponding general population. However, it is not significantly affected by the occurrence of sepsis, delirium, and acute kidney injury during intensive care unit admission when compared with that of critically ill patient control groups. High-quality studies are necessary to quantify the health-related quality of life among intensive care unit survivors.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (115) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)