Abstract
Rev Bras Ter Intensiva. 2022;34(1):87-95
DOI 10.5935/0103-507x.20220003-en
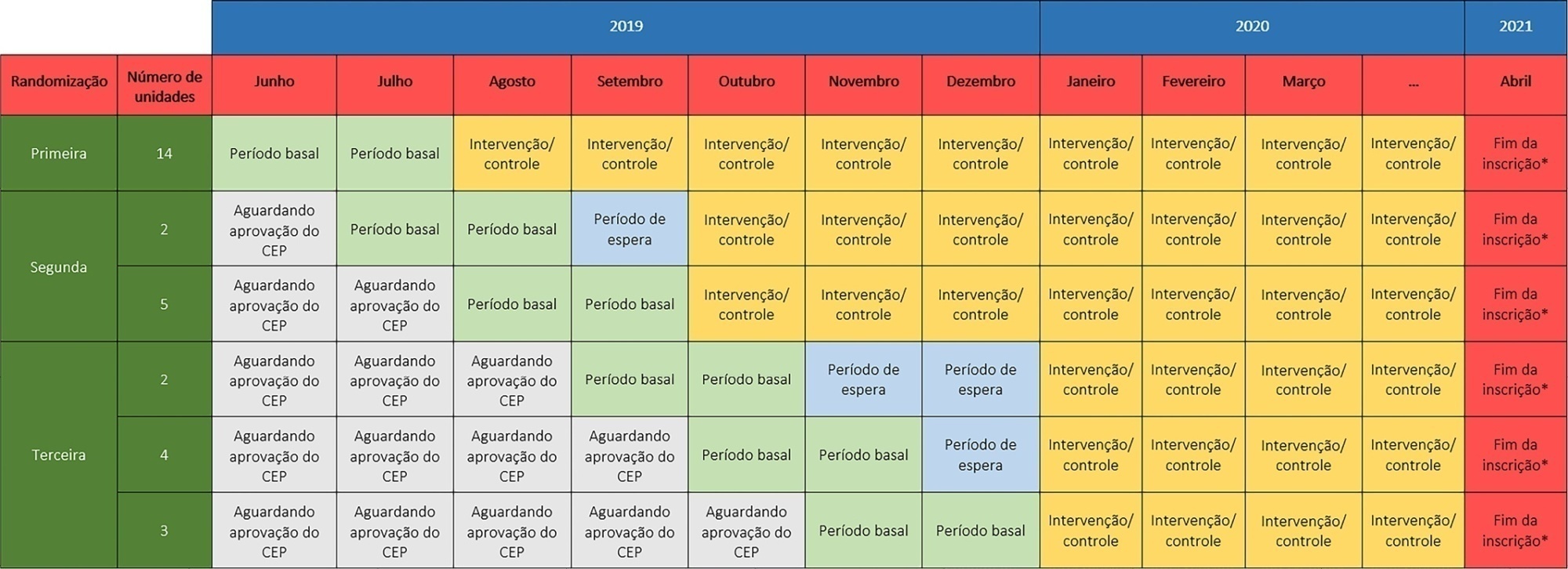
The TELE-critical Care verSus usual Care On ICU PErformance (TELESCOPE) trial aims to assess whether a complex telemedicine intervention in intensive care units, which focuses on daily multidisciplinary rounds performed by remote intensivists, will reduce intensive care unit length of stay compared to usual care.
The TELESCOPE trial is a national, multicenter, controlled, open label, cluster randomized trial. The study tests the effectiveness of daily multidisciplinary rounds conducted by an intensivist through telemedicine in Brazilian intensive care units. The protocol was approved by the local Research Ethics Committee of the coordinating study center and by the local Research Ethics Committee from each of the 30 intensive care units, following Brazilian legislation. The trial is registered with ClinicalTrials. gov (NCT03920501). The primary outcome is intensive care unit length of stay, which will be analyzed accounting for the baseline period and cluster structure of the data and adjusted by prespecified covariates. Secondary exploratory outcomes included intensive care unit performance classification, in-hospital mortality, incidence of nosocomial infections, ventilator-free days at 28 days, rate of patients receiving oral or enteral feeding, rate of patients under light sedation or alert and calm, and rate of patients under normoxemia.
According to the trial’s best practice, we report our statistical analysis prior to locking the database and beginning analyses. We anticipate that this reporting practice will prevent analysis bias and improve the interpretation of the reported results.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)