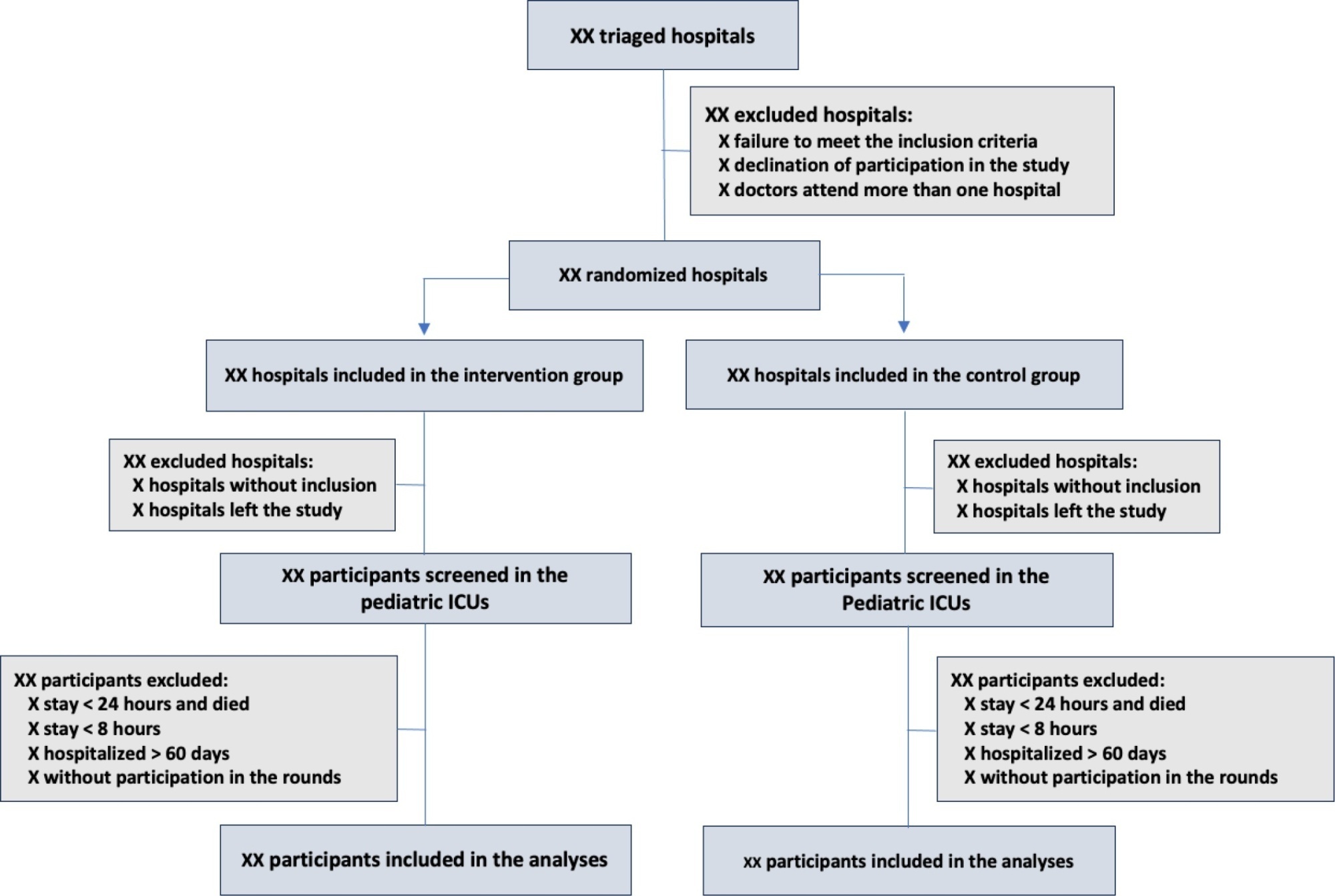
The objective of this study is to present the protocol of a cluster randomized clinical trial to be conducted through the TeleICU project – Qualification of Intensive Care by Telemedicine. The study will consist of a cluster randomized clinical trial, open label, in pediatric intensive care units, with an allocation ratio of 1:1, to compare the intervention group (support of Telemedicine for patients admitted to the pediatric intensive care unit) with a control group (pediatric intensive care unit usual care). The study proposed to select 16 pediatric intensive care units, including 100 participants per site, with a total of 1,600 participants. The intervention group will receive telerounds from Monday to Friday and will have specialists and continuing education activities available. The primary outcome measure will be the length of stay in the pediatric intensive care unit, defined as the difference between the date of discharge of the participant and the date of admission to the intensive care unit. The secondary outcomes will be mortality rate, invasive mechanical ventilation-free days, days using antibiotics, days using vasoactive drugs and days using sedoanalgesia. This study will be conducted in accordance with Resolution 466/12 of the National Health Council, with approval by the Research Ethics Committee of the institutions involved. The present study has the potential to reproduce studies on Telemedicine in intensive care and may make important contributions to care in intensive care units in Brazil and other settings. If Telemedicine shows positive clinical care results compared to conventional treatment, more pediatric patients may benefit.
ClinicalTrials.gov registry: NCT05260710
Search
Search in:


Comments