Abstract
Crit Care Sci. 2024;36:e20240208en
DOI 10.62675/2965-2774.20240208-en
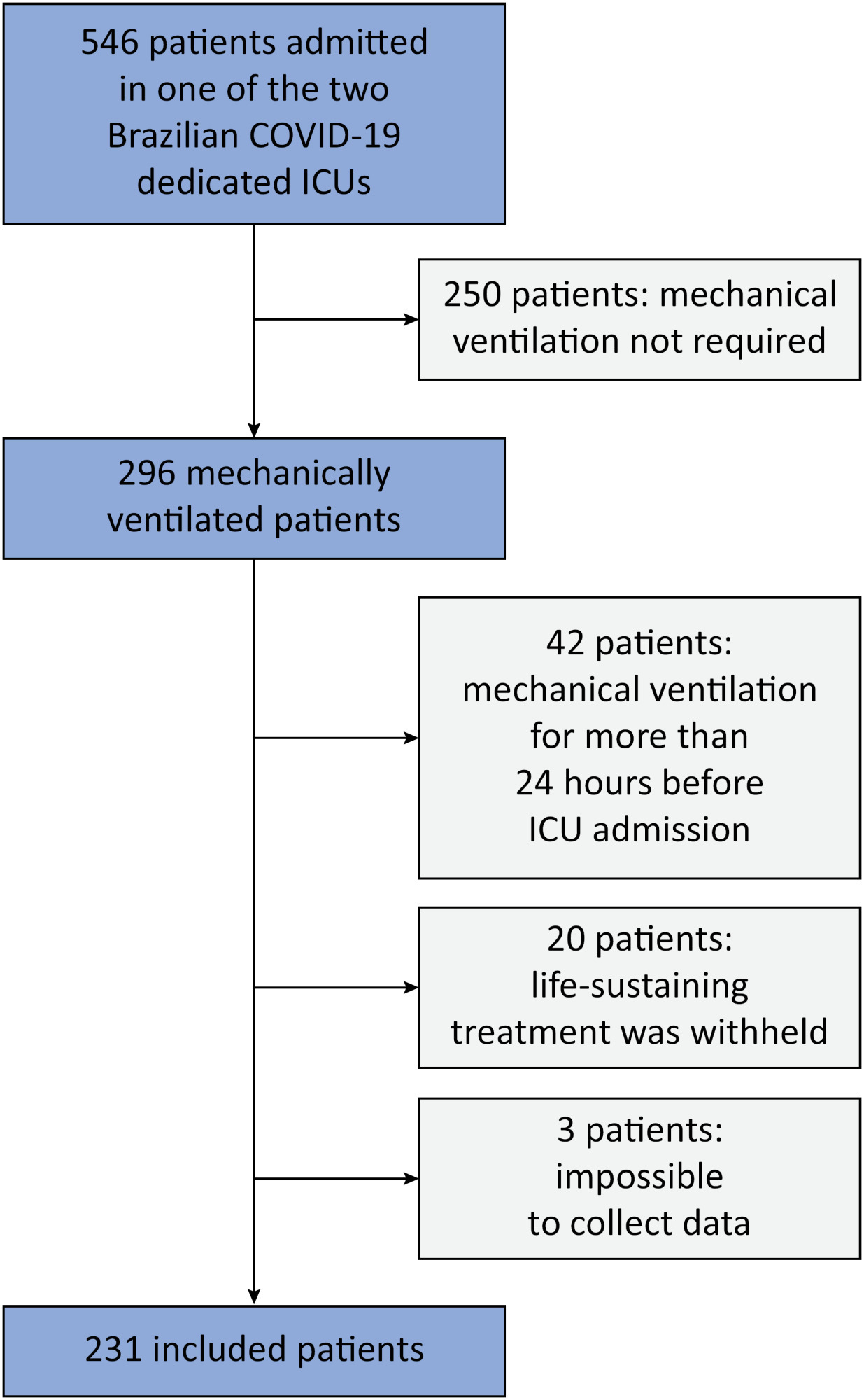
To evaluate the association between driving pressure and tidal volume based on predicted body weight and mortality in a cohort of patients with acute respiratory distress syndrome caused by COVID-19.
This was a prospective, observational study that included patients with acute respiratory distress syndrome due to COVID-19 admitted to two intensive care units. We performed multivariable analyses to determine whether driving pressure and tidal volume/kg predicted body weight on the first day of mechanical ventilation, as independent variables, are associated with hospital mortality.
We included 231 patients. The mean age was 64 (53 - 74) years, and the mean Simplified Acute and Physiology Score 3 score was 45 (39 - 54). The hospital mortality rate was 51.9%. Driving pressure was independently associated with hospital mortality (odds ratio 1.21, 95%CI 1.04 - 1.41 for each cm H2O increase in driving pressure, p = 0.01). Based on a double stratification analysis, we found that for the same level of tidal volume/kg predicted body weight, the risk of hospital death increased with increasing driving pressure. However, changes in tidal volume/kg predicted body weight were not associated with mortality when they did not lead to an increase in driving pressure.
In patients with acute respiratory distress syndrome caused by COVID-19, exposure to higher driving pressure, as opposed to higher tidal volume/kg predicted body weight, is associated with greater mortality. These results suggest that driving pressure might be a primary target for lung-protective mechanical ventilation in these patients.
Abstract
Crit Care Sci. 2023;35(4):386-393
DOI 10.5935/2965-2774.20230190-pt
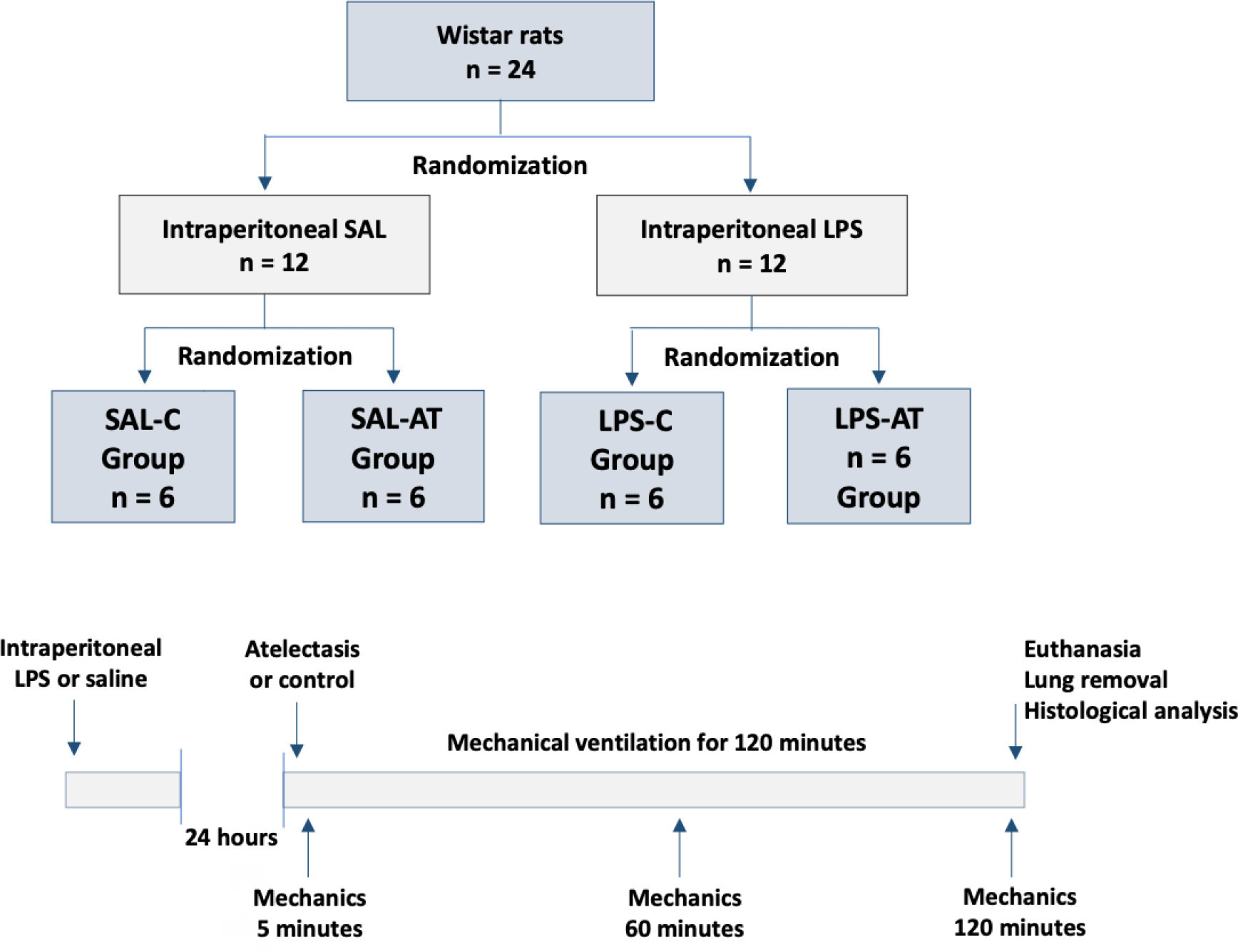
To assess the effect of atelectasis during mechanical ventilation on the periatelectatic and normal lung regions in a model of atelectasis in rats with acute lung injury induced by lipopolysaccharide.
Twenty-four rats were randomized into the following four groups, each with 6 animals: the Saline-Control Group, Lipopolysaccharide Control Group, Saline-Atelectasis Group, and Lipopolysaccharide Atelectasis Group. Acute lung injury was induced by intraperitoneal injection of lipopolysaccharide. After 24 hours, atelectasis was induced by bronchial blocking. The animals underwent mechanical ventilation for two hours with protective parameters, and respiratory mechanics were monitored during this period. Thereafter, histologic analyses of two regions of interest, periatelectatic areas and the normally-aerated lung contralateral to the atelectatic areas, were performed.
The lung injury score was significantly higher in the Lipopolysaccharide Control Group (0.41 ± 0.13) than in the Saline Control Group (0.15 ± 0.51), p < 0.05. Periatelectatic regions showed higher lung injury scores than normally-aerated regions in both the Saline-Atelectasis (0.44 ± 0.06 x 0.27 ± 0.74 p < 0.05) and Lipopolysaccharide Atelectasis (0.56 ± 0.09 x 0.35 ± 0.04 p < 0.05) Groups. The lung injury score in the periatelectatic regions was higher in the Lipopolysaccharide Atelectasis Group (0.56 ± 0.09) than in the periatelectatic region of the Saline-Atelectasis Group (0.44 ± 0.06), p < 0.05.
Atelectasis may cause injury to the surrounding tissue after a period of mechanical ventilation with protective parameters. Its effect was more significant in previously injured lungs.
Abstract
Rev Bras Ter Intensiva. 2022;34(4):402-409
DOI 10.5935/0103-507X.20220299-en
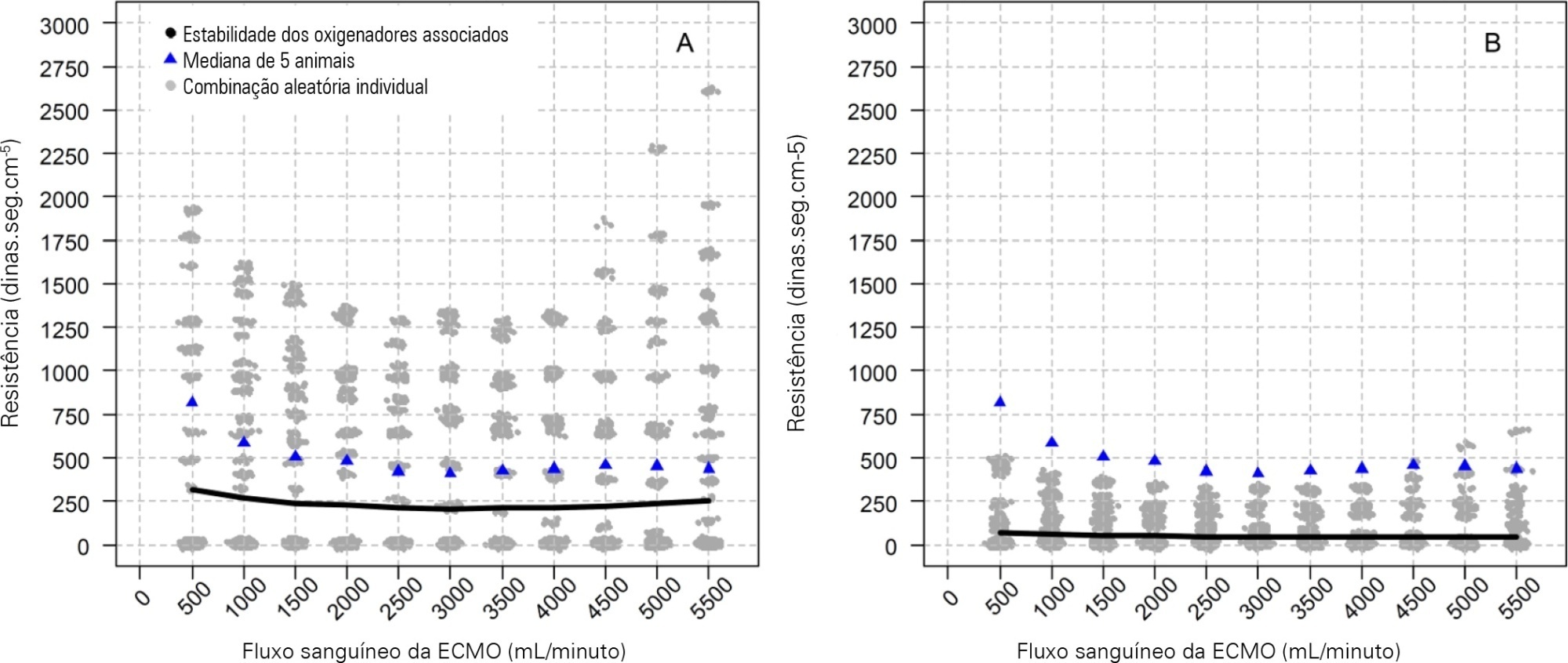
To characterize the pressures, resistances, oxygenation, and decarboxylation efficacy of two oxygenators associated in series or in parallel during venous-venous extracorporeal membrane oxygenation support.
Using the results of a swine severe respiratory failure associated with multiple organ dysfunction venous-venous extracorporeal membrane oxygenation support model and mathematical modeling, we explored the effects on oxygenation, decarboxylation and circuit pressures of in-parallel and in-series associations of oxygenators.
Five animals with a median weight of 80kg were tested. Both configurations increased the oxygen partial pressure after the oxygenators. The return cannula oxygen content was also slightly higher, but the impact on systemic oxygenation was minimal using oxygenators with a high rated flow (~ 7L/minute). Both configurations significantly reduced the systemic carbon dioxide partial pressure. As the extracorporeal membrane oxygenation blood flow increased, the oxygenator resistance decreased initially with a further increase with higher blood flows but with a small clinical impact.
Association of oxygenators in parallel or in series during venous-venous extracorporeal membrane oxygenation support provides a modest increase in carbon dioxide partial pressure removal with a slight improvement in oxygenation. The effect of oxygenator associations on extracorporeal circuit pressures is minimal.
Abstract
Rev Bras Ter Intensiva. 2022;34(4):433-442
DOI 10.5935/0103-507X.20210037-en
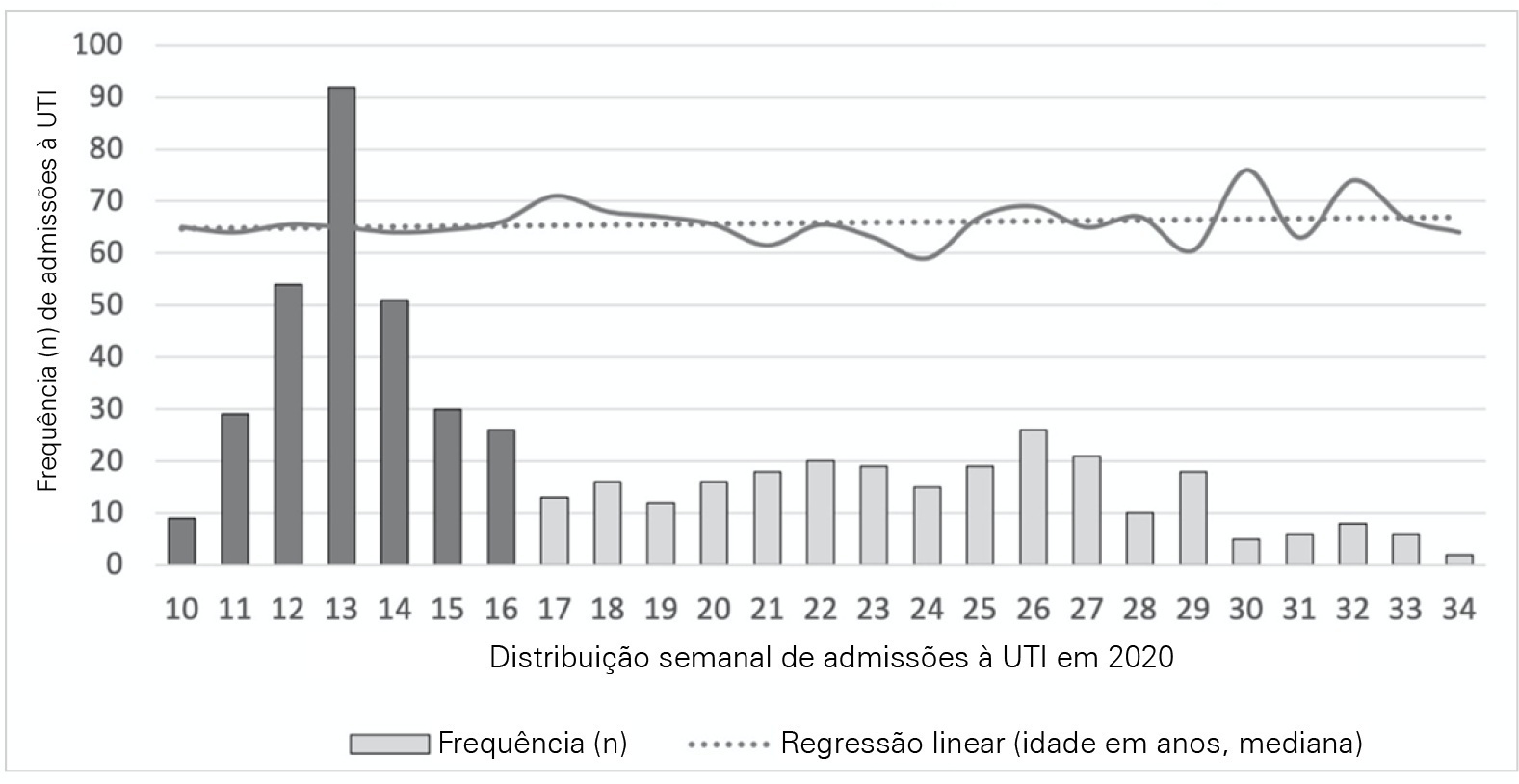
To analyze and compare COVID-19 patient characteristics, clinical management and outcomes between the peak and plateau periods of the first pandemic wave in Portugal.
This was a multicentric ambispective cohort study including consecutive severe COVID-19 patients between March and August 2020 from 16 Portuguese intensive care units. The peak and plateau periods, respectively, weeks 10 - 16 and 17 - 34, were defined.
Five hundred forty-one adult patients with a median age of 65 [57 - 74] years, mostly male (71.2%), were included. There were no significant differences in median age (p = 0.3), Simplified Acute Physiology Score II (40 versus 39; p = 0.8), partial arterial oxygen pressure/fraction of inspired oxygen ratio (139 versus 136; p = 0.6), antibiotic therapy (57% versus 64%; p = 0.2) at admission, or 28-day mortality (24.4% versus 22.8%; p = 0.7) between the peak and plateau periods. During the peak period, patients had fewer comorbidities (1 [0 - 3] versus 2 [0 - 5]; p = 0.002) and presented a higher use of vasopressors (47% versus 36%; p < 0.001) and invasive mechanical ventilation (58.1 versus 49.2%; p < 0.001) at admission, prone positioning (45% versus 36%; p = 0.04), and hydroxychloroquine (59% versus 10%; p < 0.001) and lopinavir/ritonavir (41% versus 10%; p < 0.001) prescriptions. However, a greater use of high-flow nasal cannulas (5% versus 16%, p < 0.001) on admission, remdesivir (0.3% versus 15%; p < 0.001) and corticosteroid (29% versus 52%, p < 0.001) therapy, and a shorter ICU length of stay (12 days versus 8, p < 0.001) were observed during the plateau.
There were significant changes in patient comorbidities, intensive care unit therapies and length of stay between the peak and plateau periods of the first COVID-19 wave.
Abstract
Rev Bras Ter Intensiva. 2021;33(1):75-81
DOI 10.5935/0103-507X.20210007
To detect early respiratory and hemodynamic instability to characterize pulmonary impairment in patients with severe COVID-19.
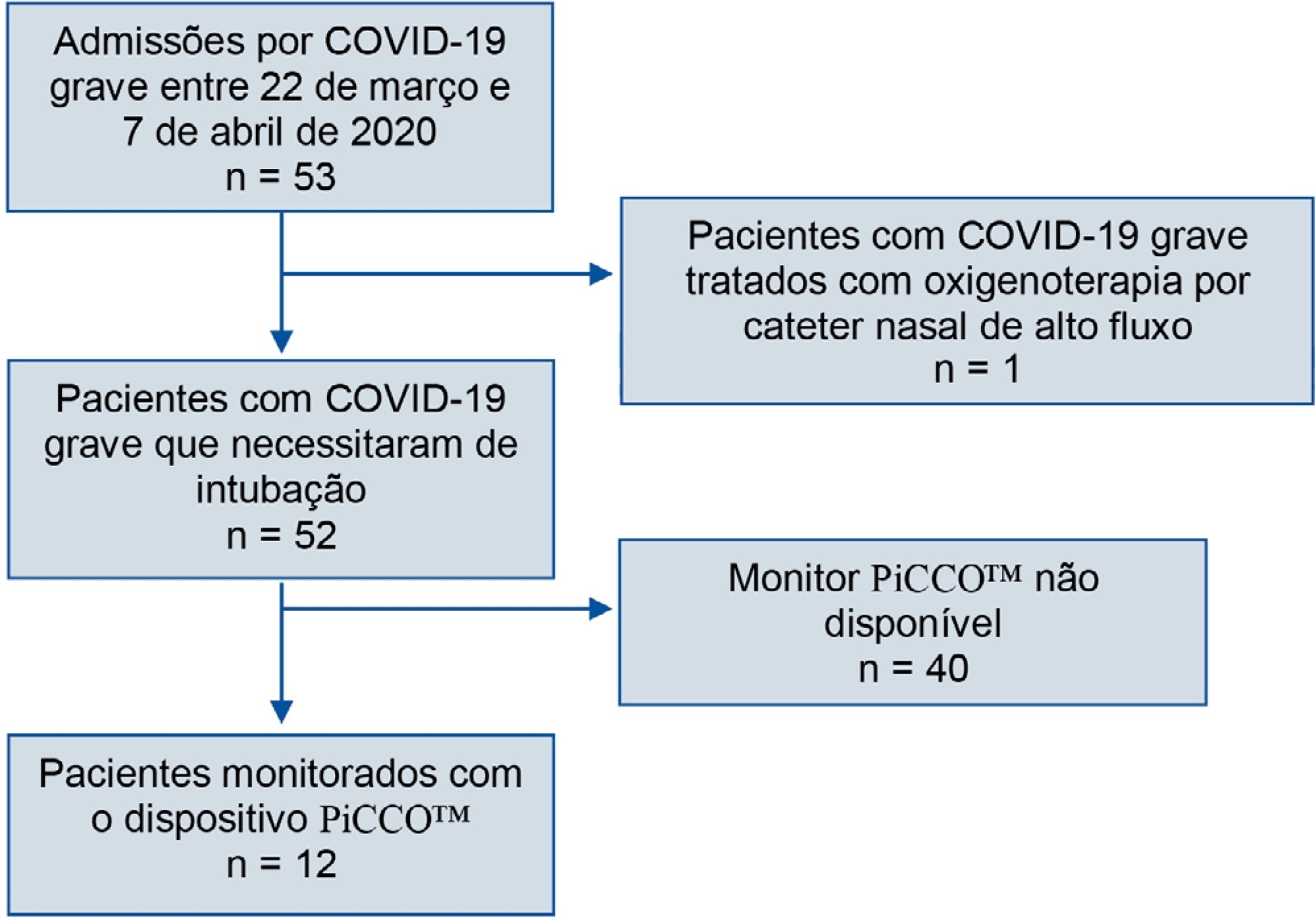
We retrospectively analyzed data collected from COVID-19 patients suffering from acute respiratory failure requiring intubation and mechanical ventilation. We used transpulmonary thermodilution assessment with a PiCCO™ device. We collected demographic, respiratory, hemodynamic and echocardiographic data within the first 48 hours after admission. Descriptive statistics were used to summarize the data.
Fifty-three patients with severe COVID-19 were admitted between March 22nd and April 7th. Twelve of them (22.6%) were monitored with a PiCCO™ device. Upon admission, the global-end diastolic volume indexed was normal (mean 738.8mL ± 209.2) and moderately increased at H48 (879mL ± 179), and the cardiac index was subnormal (2.84 ± 0.65). All patients showed extravascular lung water over 8mL/kg on admission (17.9 ± 8.9). We did not identify any argument for cardiogenic failure.
In the case of severe COVID-19 pneumonia, hemodynamic and respiratory presentation is consistent with pulmonary edema without evidence of cardiogenic origin, favoring the diagnosis of acute respiratory distress syndrome.
Abstract
Rev Bras Ter Intensiva. 2019;31(2):113-121
DOI 10.5935/0103-507X.20190018
To describe (1) the energy transfer from the ventilator to the lungs, (2) the match between venous-venous extracorporeal membrane oxygenation (ECMO) oxygen transfer and patient oxygen consumption (VO2), (3) carbon dioxide removal with ECMO, and (4) the potential effect of systemic venous oxygenation on pulmonary artery pressure.
Mathematical modeling approach with hypothetical scenarios using computer simulation.
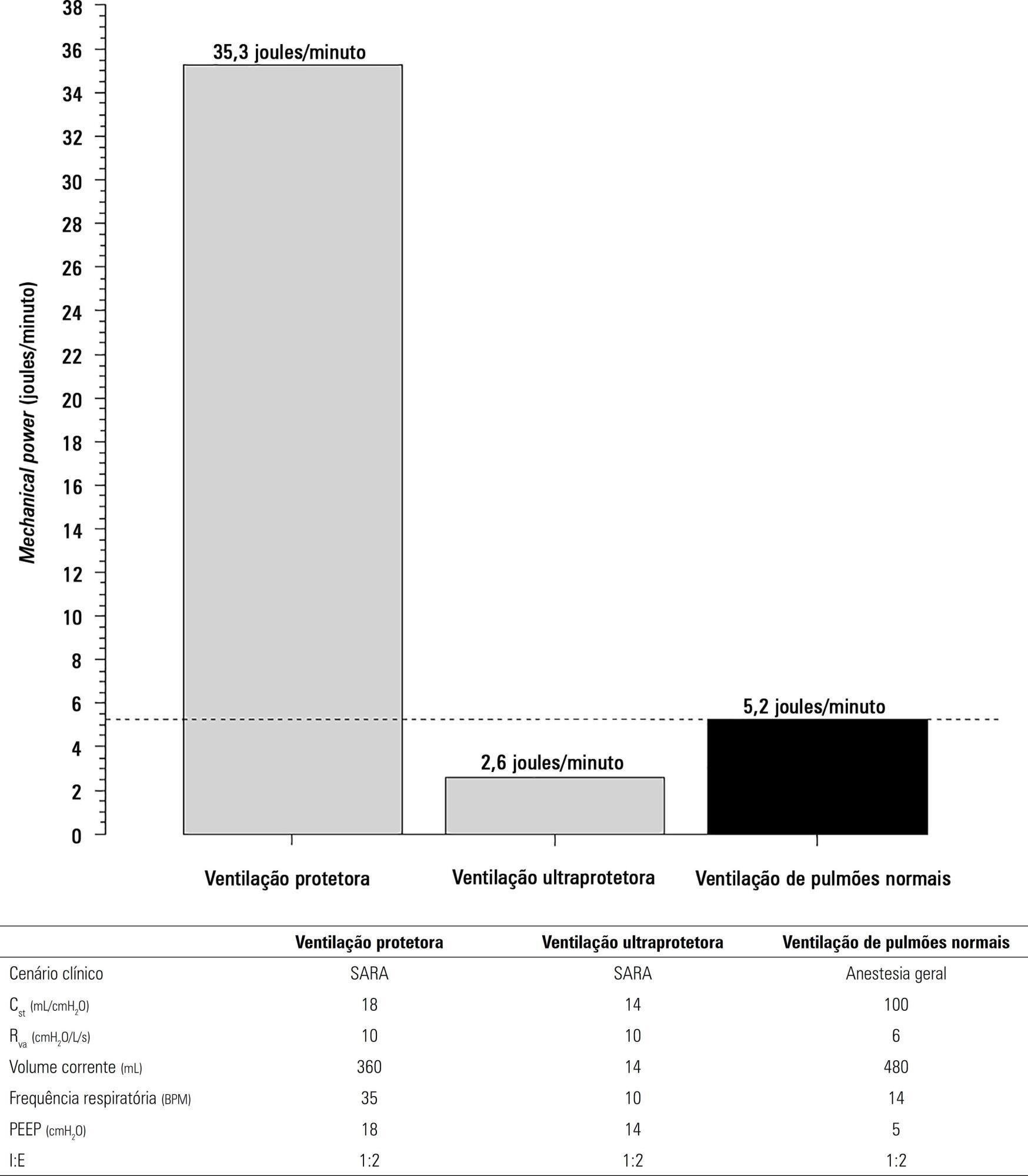
The transition from protective ventilation to ultraprotective ventilation in a patient with severe acute respiratory distress syndrome and a static respiratory compliance of 20mL/cm H2O reduced the energy transfer from the ventilator to the lungs from 35.3 to 2.6 joules/minute. A hypothetical patient, hyperdynamic and slightly anemic with VO2 = 200mL/minute, can reach an arterial oxygen saturation of 80%, while maintaining the match between the oxygen transfer by ECMO and the VO2 of the patient. Carbon dioxide is easily removed, and normal PaCO2 is easily reached. Venous blood oxygenation through the ECMO circuit may drive the PO2 stimulus of pulmonary hypoxic vasoconstriction to normal values.
Ultraprotective ventilation largely reduces the energy transfer from the ventilator to the lungs. Severe hypoxemia on venous-venous-ECMO support may occur despite the matching between the oxygen transfer by ECMO and the VO2 of the patient. The normal range of PaCO2 is easy to reach. Venous-venous-ECMO support potentially relieves hypoxic pulmonary vasoconstriction.
Abstract
Rev Bras Ter Intensiva. 2017;29(4):427-435
DOI 10.5935/0103-507X.20170067
To compare the effects of high-frequency oscillatory ventilation and conventional protective mechanical ventilation associated with the prone position on oxygenation, histology and pulmonary oxidative damage in an experimental model of acute lung injury.
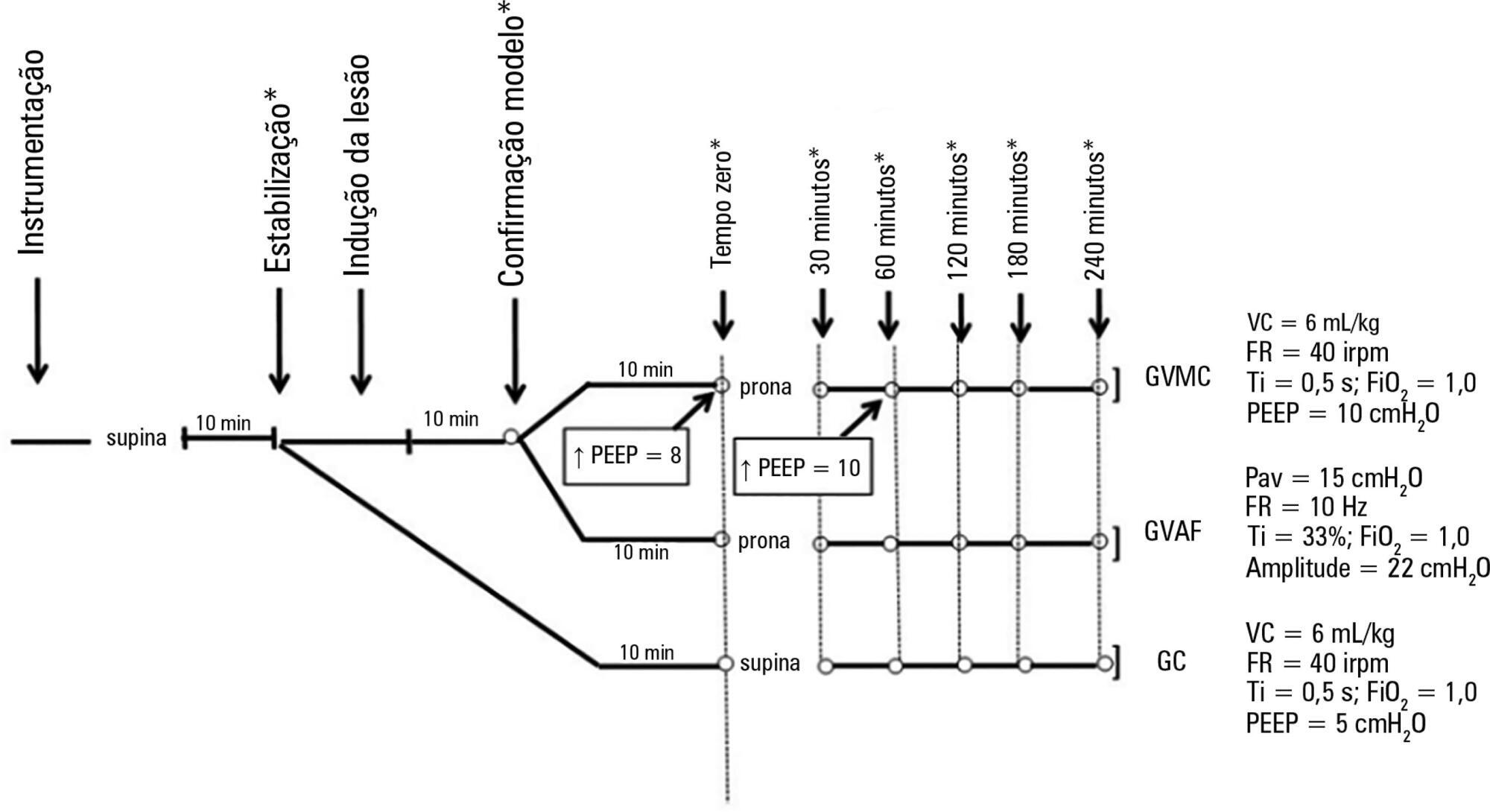
Forty-five rabbits with tracheostomy and vascular access were underwent mechanical ventilation. Acute lung injury was induced by tracheal infusion of warm saline. Three experimental groups were formed: healthy animals + conventional protective mechanical ventilation, supine position (Control Group; n = 15); animals with acute lung injury + conventional protective mechanical ventilation, prone position (CMVG; n = 15); and animals with acute lung injury + high-frequency oscillatory ventilation, prone position (HFOG; n = 15). Ten minutes after the beginning of the specific ventilation of each group, arterial gasometry was collected, with this timepoint being called time zero, after which the animal was placed in prone position and remained in this position for 4 hours. Oxidative stress was evaluated by the total antioxidant performance assay. Pulmonary tissue injury was determined by histopathological score. The level of significance was 5%.
Both groups with acute lung injury showed worsening of oxygenation after induction of injury compared with the Control Group. After 4 hours, there was a significant improvement in oxygenation in the HFOG group compared with CMVG. Analysis of total antioxidant performance in plasma showed greater protection in HFOG. HFOG had a lower histopathological lesion score in lung tissue than CMVG.
High-frequency oscillatory ventilation, associated with prone position, improves oxygenation and attenuates oxidative damage and histopathological lung injury compared with conventional protective mechanical ventilation.
Abstract
Rev Bras Ter Intensiva. 2017;29(3):354-363
DOI 10.5935/0103-507X.20170044
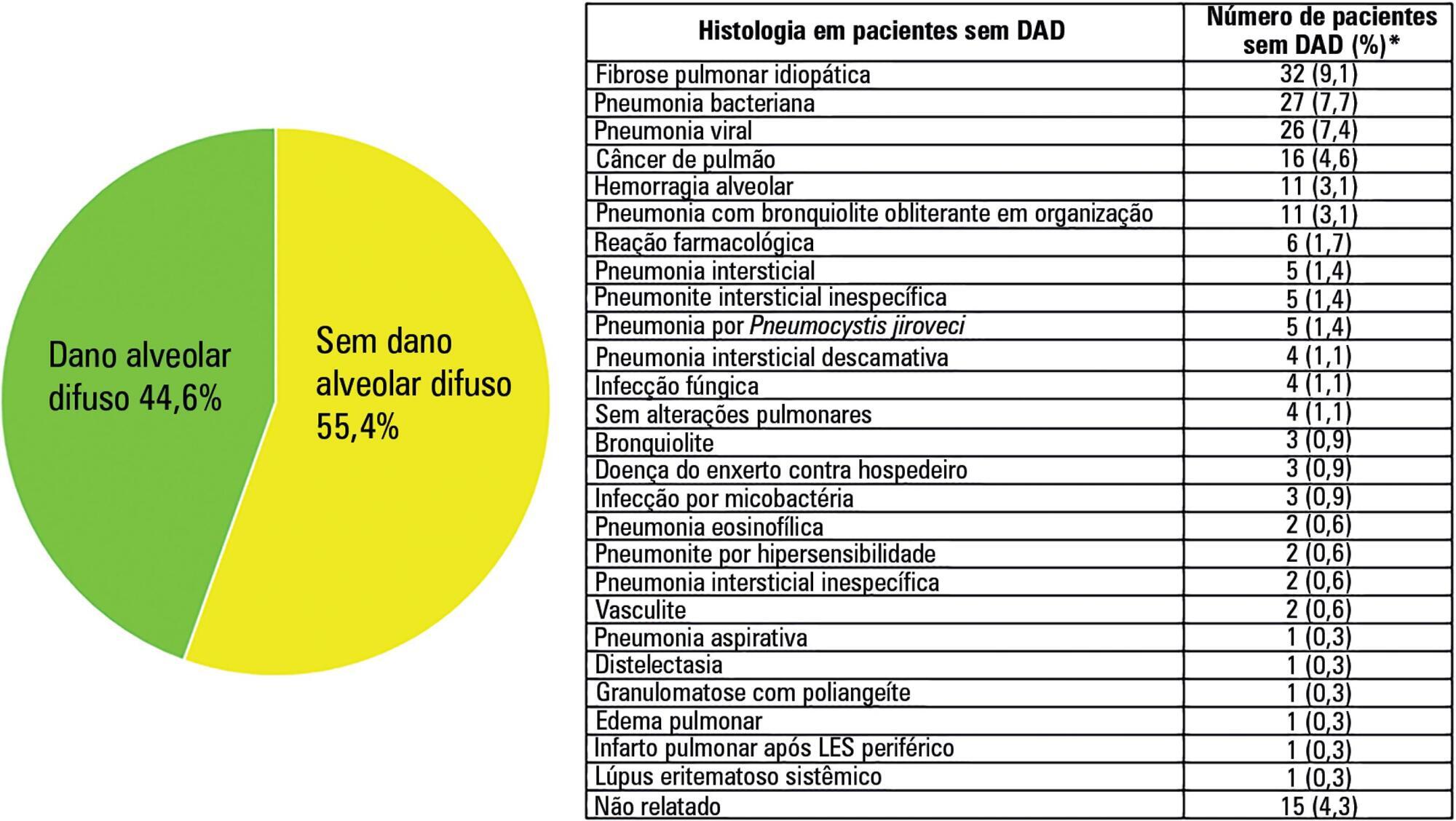
Acute respiratory distress syndrome is a challenging entity for the intensivist. The pathological hallmark of the acute phase is diffuse alveolar damage, which is present in approximately half of living patients with acute respiratory distress syndrome. It is clear that respiratory support for acute respiratory distress syndrome has gradually been improving over recent decades. However, it is also evident that these procedures are beneficial, as they reduce lung injury and keep the patient alive. This could be interpreted as a time-gaining strategy until the trigger or causal or risk factor improves, the inflammatory storm decreases and the lung heals. However, all except two pharmacological treatments (neuromuscular blockers and steroids) were unable to improve the acute respiratory distress syndrome outcome. The hypothesis that pharmacological negative results may be explained by the histological heterogeneity of acute respiratory distress syndrome has been supported by the recent demonstration that acute respiratory distress syndrome with diffuse alveolar damage constitutes a specific clinical-pathological entity. Given that diffuse alveolar damage is a pathological diagnosis and that open lung biopsy (the most common technique to obtain lung tissue) has several side effects, it is necessary to develop surrogate biomarkers for diffuse alveolar damage. The aim of this narrative review is to address the following three topics related to acute respiratory distress syndrome: (a) the relationship between acute respiratory distress syndrome and diffuse alveolar damage, (b) how diffuse alveolar damage could be surrogated in the clinical setting and (c) how enrichment in diffuse alveolar damage may improve the results of pharmacological clinical trials tried out on patients with acute respiratory distress syndrome.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)