Abstract
Rev Bras Ter Intensiva. 2022;34(4):433-442
DOI 10.5935/0103-507X.20210037-en
To analyze and compare COVID-19 patient characteristics, clinical management and outcomes between the peak and plateau periods of the first pandemic wave in Portugal.
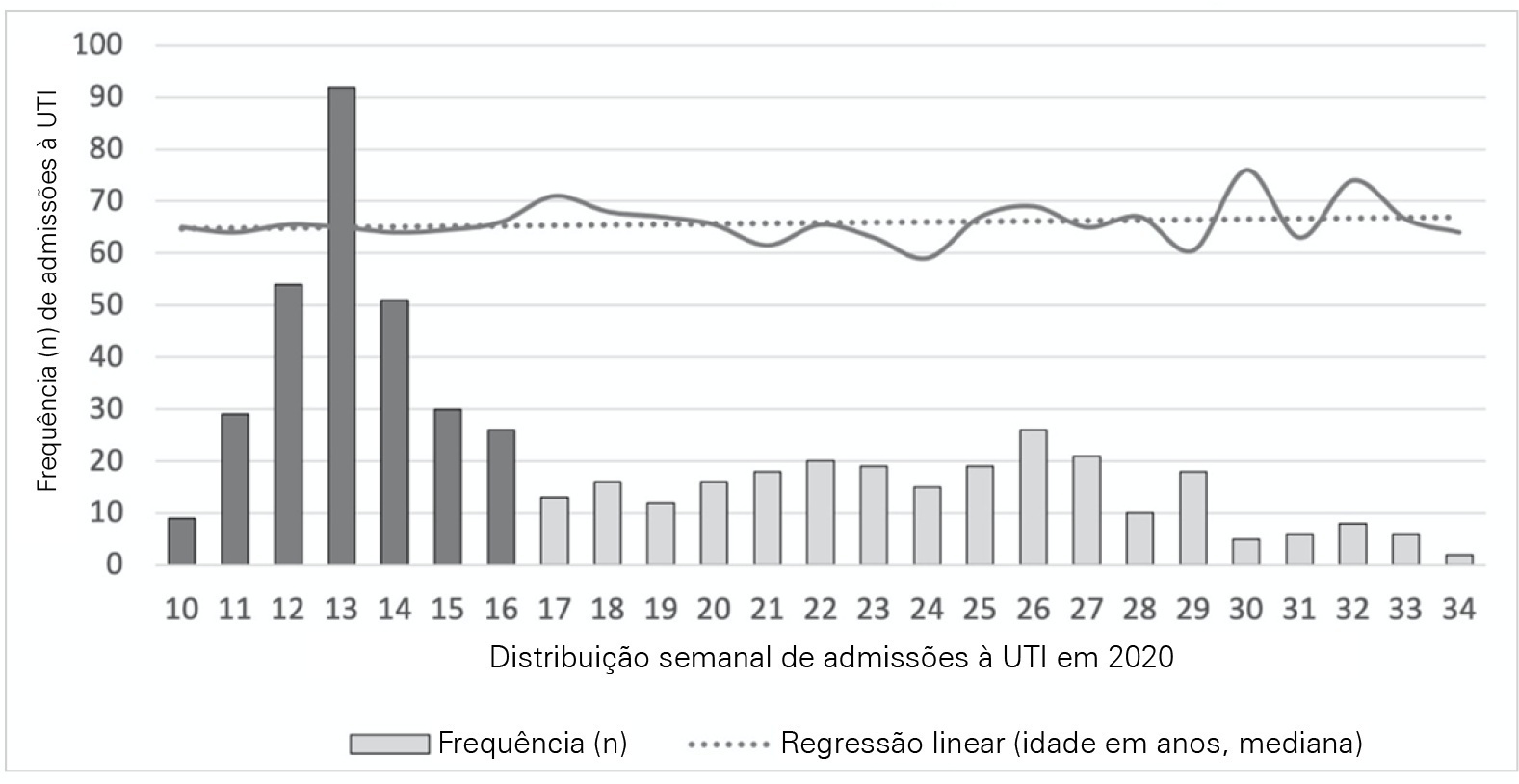
This was a multicentric ambispective cohort study including consecutive severe COVID-19 patients between March and August 2020 from 16 Portuguese intensive care units. The peak and plateau periods, respectively, weeks 10 - 16 and 17 - 34, were defined.
Five hundred forty-one adult patients with a median age of 65 [57 - 74] years, mostly male (71.2%), were included. There were no significant differences in median age (p = 0.3), Simplified Acute Physiology Score II (40 versus 39; p = 0.8), partial arterial oxygen pressure/fraction of inspired oxygen ratio (139 versus 136; p = 0.6), antibiotic therapy (57% versus 64%; p = 0.2) at admission, or 28-day mortality (24.4% versus 22.8%; p = 0.7) between the peak and plateau periods. During the peak period, patients had fewer comorbidities (1 [0 - 3] versus 2 [0 - 5]; p = 0.002) and presented a higher use of vasopressors (47% versus 36%; p < 0.001) and invasive mechanical ventilation (58.1 versus 49.2%; p < 0.001) at admission, prone positioning (45% versus 36%; p = 0.04), and hydroxychloroquine (59% versus 10%; p < 0.001) and lopinavir/ritonavir (41% versus 10%; p < 0.001) prescriptions. However, a greater use of high-flow nasal cannulas (5% versus 16%, p < 0.001) on admission, remdesivir (0.3% versus 15%; p < 0.001) and corticosteroid (29% versus 52%, p < 0.001) therapy, and a shorter ICU length of stay (12 days versus 8, p < 0.001) were observed during the plateau.
There were significant changes in patient comorbidities, intensive care unit therapies and length of stay between the peak and plateau periods of the first COVID-19 wave.
Abstract
Rev Bras Ter Intensiva. 2020;32(3):354-362
DOI 10.5935/0103-507X.20200063
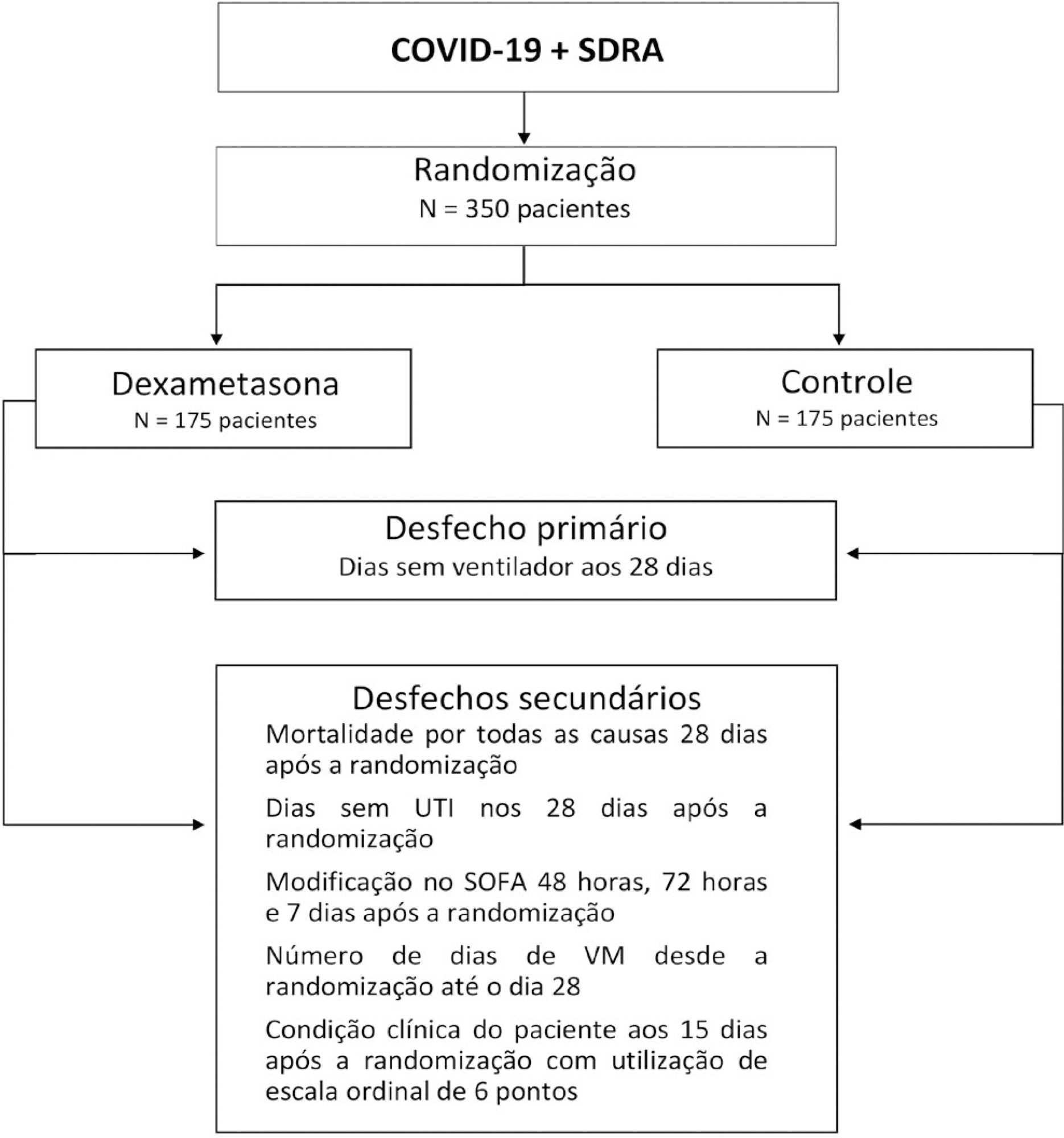
The infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spreads worldwide and is considered a pandemic. The most common manifestation of SARS-CoV-2 infection (coronavirus disease 2019 - COVID-19) is viral pneumonia with varying degrees of respiratory compromise and up to 40% of hospitalized patients might develop acute respiratory distress syndrome. Several clinical trials evaluated the role of corticosteroids in non-COVID-19 acute respiratory distress syndrome with conflicting results. We designed a trial to evaluate the effectiveness of early intravenous dexamethasone administration on the number of days alive and free of mechanical ventilation within 28 days after randomization in adult patients with moderate or severe acute respiratory distress syndrome due to confirmed or probable COVID-19.
This is a pragmatic, prospective, randomized, stratified, multicenter, open-label, controlled trial including 350 patients with early-onset (less than 48 hours before randomization) moderate or severe acute respiratory distress syndrome, defined by the Berlin criteria, due to COVID-19. Eligible patients will be randomly allocated to either standard treatment plus dexamethasone (Intervention Group) or standard treatment without dexamethasone (Control Group). Patients in the intervention group will receive dexamethasone 20mg intravenous once daily for 5 days, followed by dexamethasone 10mg IV once daily for additional 5 days or until intensive care unit discharge, whichever occurs first. The primary outcome is ventilator-free days within 28 days after randomization, defined as days alive and free from invasive mechanical ventilation. Secondary outcomes are all-cause mortality rates at day 28, evaluation of the clinical status at day 15 assessed with a 6-level ordinal scale, mechanical ventilation duration from randomization to day 28, Sequential Organ Failure Assessment Score evaluation at 48 hours, 72 hours and 7 days and intensive care unit -free days within 28.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (115) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)