Abstract
Rev Bras Ter Intensiva. 2020;32(3):348-353
DOI 10.5935/0103-507X.20200062
A novel coronavirus emerged this year as a cause of viral pneumonia. The main characteristics of the virus are rapid transmission, high contagion capacity and potential severity. The objective of this case series study is to describe the clinical characteristics of patients with confirmed coronavirus disease (COVID-19) admitted to different intensive care units in Argentina for mechanical ventilation.
A descriptive, prospective, multicenter case series study was conducted between April 1 and May 8, 2020. Data from patients older than 18 years who were admitted to the intensive care unit for mechanical ventilation for acute respiratory failure with a positive diagnosis of COVID-19 were included.
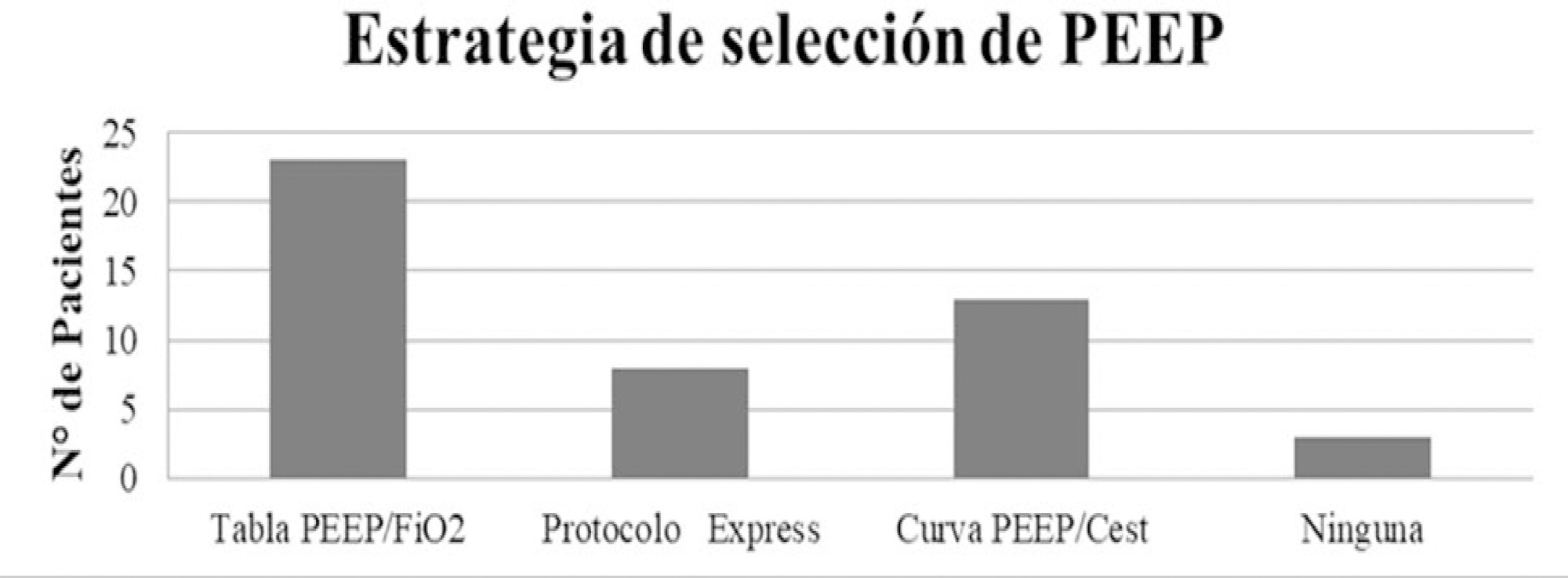
The variables for 47 patients from 31 intensive care units were recorded: 78.7% were men (median age of 61 years), with a SAPS II score of 43 and a Charlson index score of 3. The initial ventilatory mode was volume control - continuous mandatory ventilation with a tidal volume less than 8mL/kg in 100% of cases, with a median positive end-expiratory pressure of 10.5cmH2O. At the end of the study, 29 patients died, 8 were discharged, and 10 remained hospitalized. The SAPS II score was higher among patients who died (p = 0.046). Charlson comorbidity index was associated with higher mortality (OR = 2.27, 95% CI 1.13 - 4.55, p = 0.02).
Patients with COVID-19 and on mechanical ventilation in this series presented clinical variables similar to those described to date in other international reports. Our findings provide data that may predict outcomes.
Abstract
Rev Bras Ter Intensiva. 2020;32(3):354-362
DOI 10.5935/0103-507X.20200063
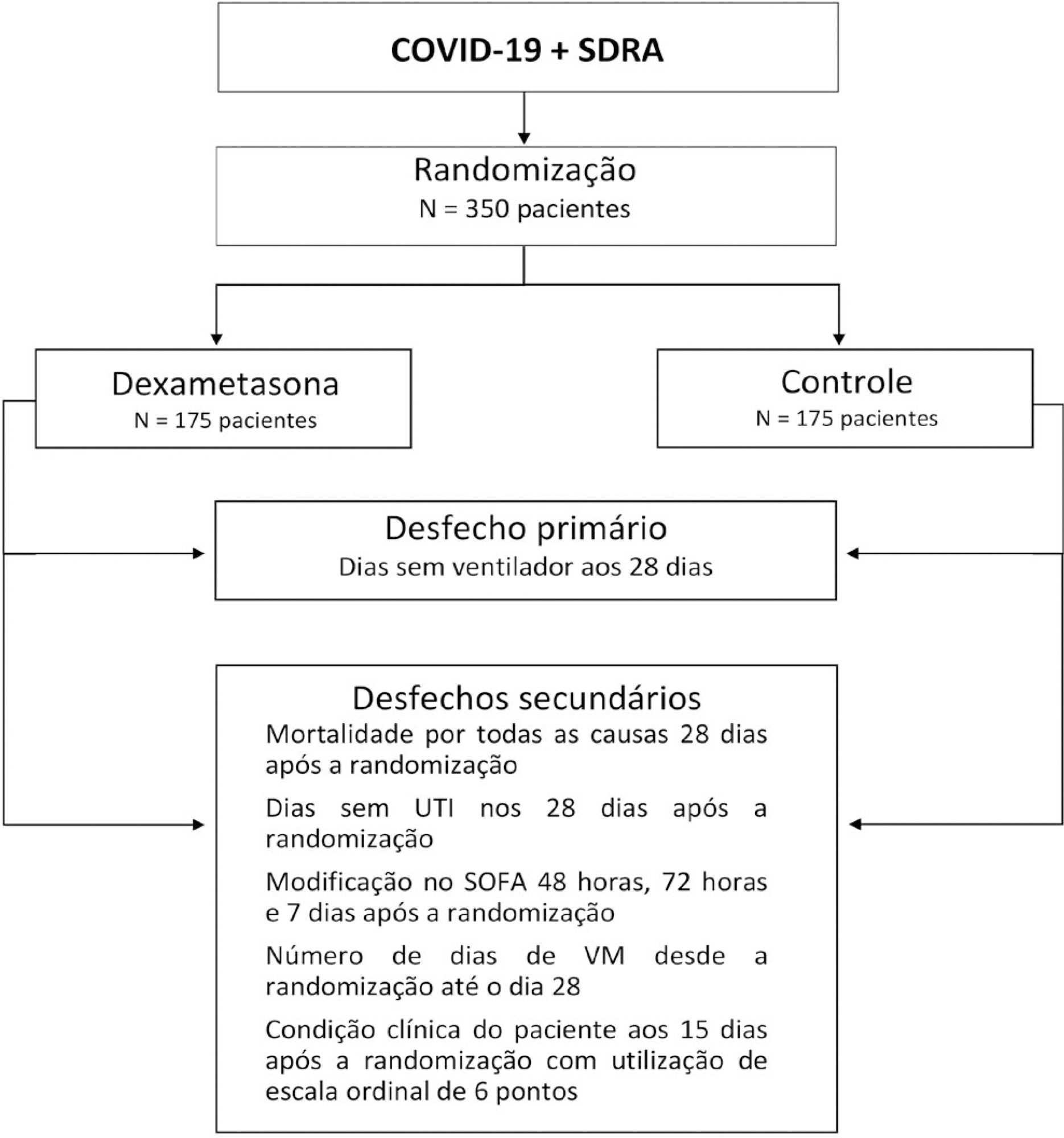
The infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spreads worldwide and is considered a pandemic. The most common manifestation of SARS-CoV-2 infection (coronavirus disease 2019 - COVID-19) is viral pneumonia with varying degrees of respiratory compromise and up to 40% of hospitalized patients might develop acute respiratory distress syndrome. Several clinical trials evaluated the role of corticosteroids in non-COVID-19 acute respiratory distress syndrome with conflicting results. We designed a trial to evaluate the effectiveness of early intravenous dexamethasone administration on the number of days alive and free of mechanical ventilation within 28 days after randomization in adult patients with moderate or severe acute respiratory distress syndrome due to confirmed or probable COVID-19.
This is a pragmatic, prospective, randomized, stratified, multicenter, open-label, controlled trial including 350 patients with early-onset (less than 48 hours before randomization) moderate or severe acute respiratory distress syndrome, defined by the Berlin criteria, due to COVID-19. Eligible patients will be randomly allocated to either standard treatment plus dexamethasone (Intervention Group) or standard treatment without dexamethasone (Control Group). Patients in the intervention group will receive dexamethasone 20mg intravenous once daily for 5 days, followed by dexamethasone 10mg IV once daily for additional 5 days or until intensive care unit discharge, whichever occurs first. The primary outcome is ventilator-free days within 28 days after randomization, defined as days alive and free from invasive mechanical ventilation. Secondary outcomes are all-cause mortality rates at day 28, evaluation of the clinical status at day 15 assessed with a 6-level ordinal scale, mechanical ventilation duration from randomization to day 28, Sequential Organ Failure Assessment Score evaluation at 48 hours, 72 hours and 7 days and intensive care unit -free days within 28.
Abstract
Rev Bras Ter Intensiva. 2020;32(3):337-347
DOI 10.5935/0103-507X.20200060
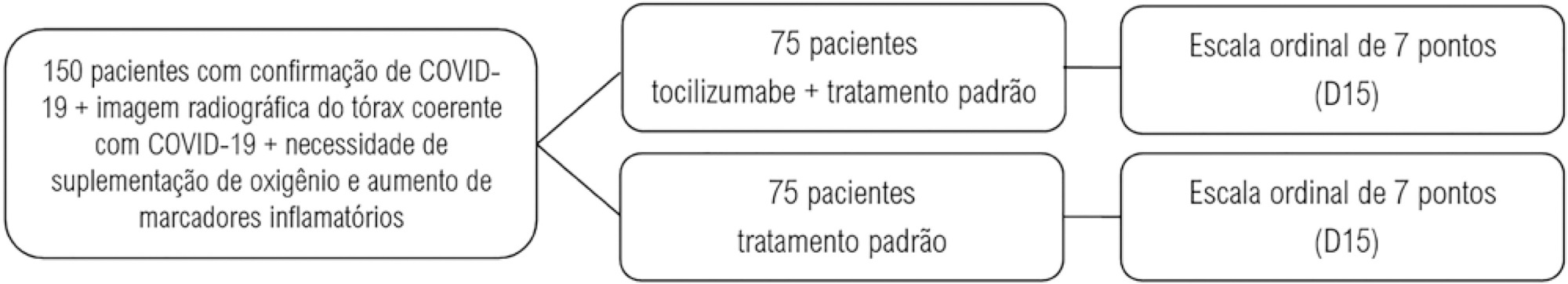
Pro-inflammatory markers play a significant role in the disease severity of patients with COVID-19. Thus, anti-inflammatory therapies are attractive agents for potentially combating the uncontrolled inflammatory cascade in these patients. We designed a trial testing tocilizumab versus standard of care intending to improve the outcomes by inhibiting interleukin-6, an important inflammatory mediator in COVID-19.
This open-label multicentre randomized controlled trial will compare clinical outcomes of tocilizumab plus standard of care versus standard of care alone in patients with moderate to severe COVID-19. Two of the following four criteria are required for protocol enrolment: D-dimer > 1,000ng/mL; C reactive protein > 5mg/dL, ferritin > 300mg/dL, and lactate dehydrogenase > upper limit of normal. The primary objective will be to compare the clinical status on day 15, as measured by a 7-point ordinal scale applied in COVID-19 trials worldwide. The primary endpoint will be assessed by an ordinal logistic regression assuming proportional odds ratios adjusted for stratification variables (age and sex).
The TOCIBRAS protocol was approved by local and central (national) ethical committees in Brazil following current national and international guidelines/directives. Each participating center had the study protocol approved by their institutional review boards before initiating protocol enrolment. The data derived from this trial will be published regardless of the results. If proven active, this strategy could alleviate the consequences of the inflammatory response in COVID-19 patients and improve their clinical outcomes.