Abstract
Crit Care Sci. 2024;36:e20240210en
DOI 10.62675/2965-2774.20240210-en
Driving pressure has been suggested to be the main driver of ventilator-induced lung injury and mortality in observational studies of acute respiratory distress syndrome. Whether a driving pressure-limiting strategy can improve clinical outcomes is unclear.
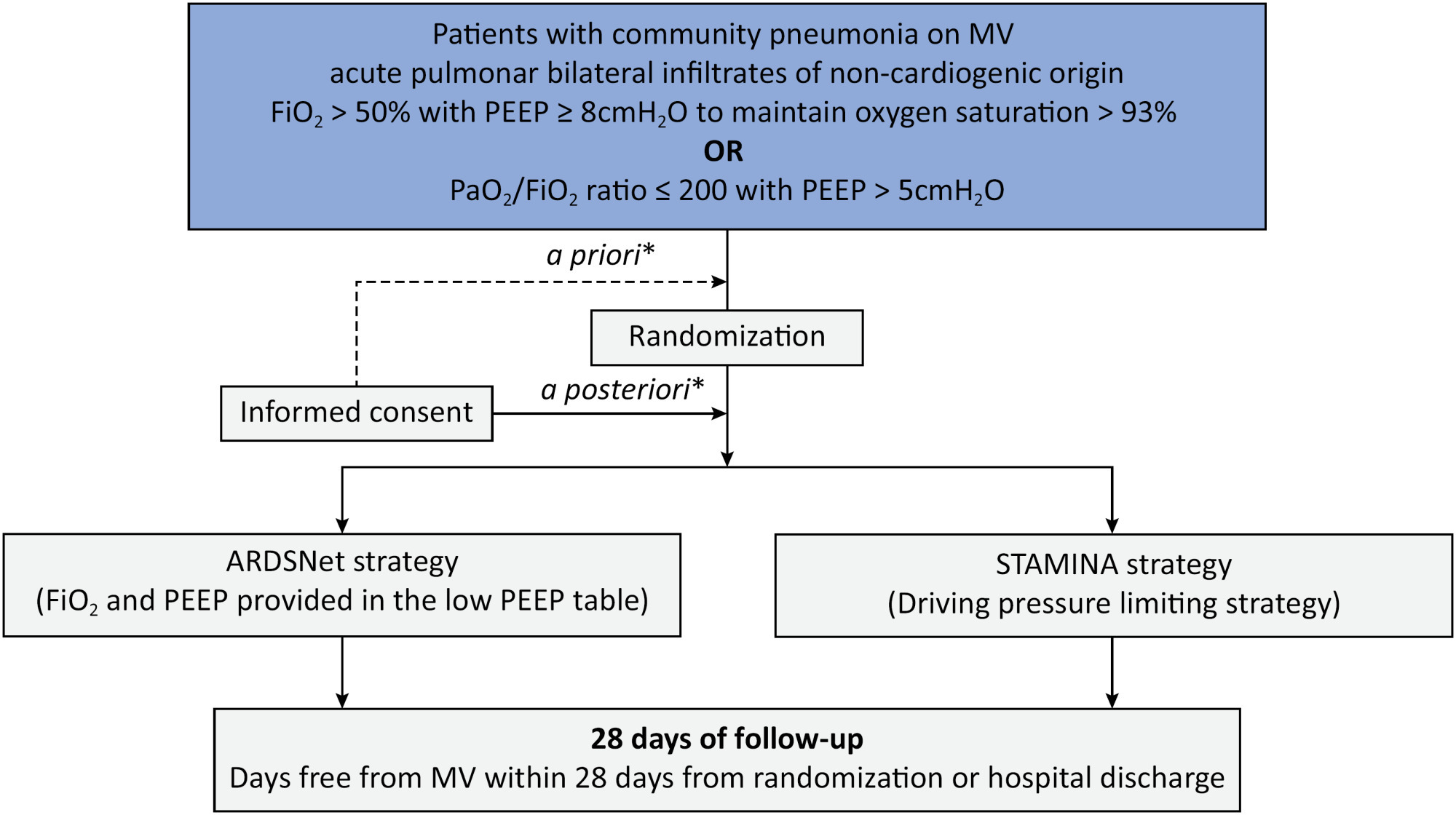
To describe the protocol and statistical analysis plan that will be used to test whether a driving pressure-limiting strategy including positive end-expiratory pressure titration according to the best respiratory compliance and reduction in tidal volume is superior to a standard strategy involving the use of the ARDSNet low-positive end-expiratory pressure table in terms of increasing the number of ventilator-free days in patients with acute respiratory distress syndrome due to community-acquired pneumonia.
The ventilator STrAtegy for coMmunIty acquired pNeumoniA (STAMINA) study is a randomized, multicenter, open-label trial that compares a driving pressure-limiting strategy to the ARDSnet low-positive end-expiratory pressure table in patients with moderate-to-severe acute respiratory distress syndrome due to community-acquired pneumonia admitted to intensive care units. We expect to recruit 500 patients from 20 Brazilian and 2 Colombian intensive care units. They will be randomized to a driving pressure-limiting strategy group or to a standard strategy using the ARDSNet low-positive end-expiratory pressure table. In the driving pressure-limiting strategy group, positive end-expiratory pressure will be titrated according to the best respiratory system compliance.
The primary outcome is the number of ventilator-free days within 28 days. The secondary outcomes are in-hospital and intensive care unit mortality and the need for rescue therapies such as extracorporeal life support, recruitment maneuvers and inhaled nitric oxide.
STAMINA is designed to provide evidence on whether a driving pressure-limiting strategy is superior to the ARDSNet low-positive end-expiratory pressure table strategy for increasing the number of ventilator-free days within 28 days in patients with moderate-to-severe acute respiratory distress syndrome. Here, we describe the rationale, design and status of the trial.
Abstract
Crit Care Sci. 2023;35(4):386-393
DOI 10.5935/2965-2774.20230190-pt
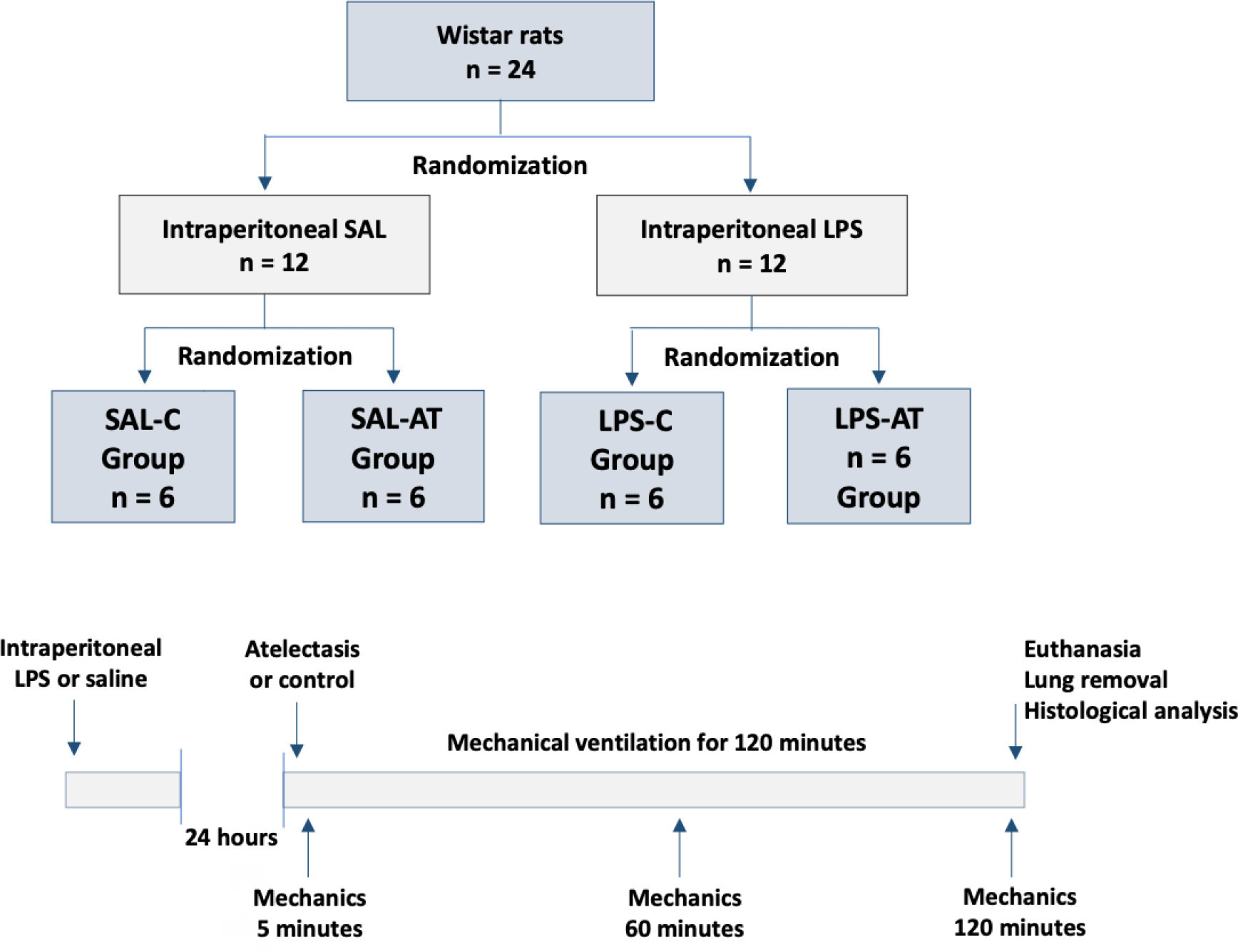
To assess the effect of atelectasis during mechanical ventilation on the periatelectatic and normal lung regions in a model of atelectasis in rats with acute lung injury induced by lipopolysaccharide.
Twenty-four rats were randomized into the following four groups, each with 6 animals: the Saline-Control Group, Lipopolysaccharide Control Group, Saline-Atelectasis Group, and Lipopolysaccharide Atelectasis Group. Acute lung injury was induced by intraperitoneal injection of lipopolysaccharide. After 24 hours, atelectasis was induced by bronchial blocking. The animals underwent mechanical ventilation for two hours with protective parameters, and respiratory mechanics were monitored during this period. Thereafter, histologic analyses of two regions of interest, periatelectatic areas and the normally-aerated lung contralateral to the atelectatic areas, were performed.
The lung injury score was significantly higher in the Lipopolysaccharide Control Group (0.41 ± 0.13) than in the Saline Control Group (0.15 ± 0.51), p < 0.05. Periatelectatic regions showed higher lung injury scores than normally-aerated regions in both the Saline-Atelectasis (0.44 ± 0.06 x 0.27 ± 0.74 p < 0.05) and Lipopolysaccharide Atelectasis (0.56 ± 0.09 x 0.35 ± 0.04 p < 0.05) Groups. The lung injury score in the periatelectatic regions was higher in the Lipopolysaccharide Atelectasis Group (0.56 ± 0.09) than in the periatelectatic region of the Saline-Atelectasis Group (0.44 ± 0.06), p < 0.05.
Atelectasis may cause injury to the surrounding tissue after a period of mechanical ventilation with protective parameters. Its effect was more significant in previously injured lungs.
Abstract
Rev Bras Ter Intensiva. 2021;33(4):572-582
DOI 10.5935/0103-507X.20210084
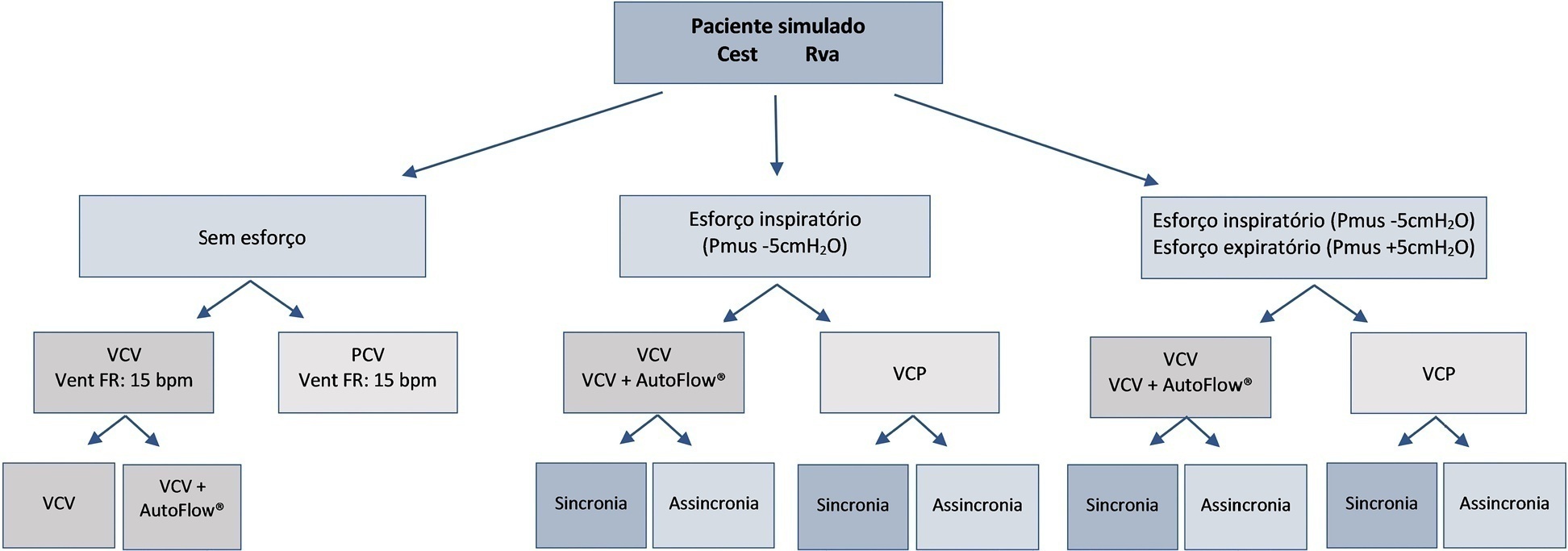
To evaluate the influences of respiratory muscle efforts and respiratory rate setting in the ventilator on tidal volume and alveolar distending pressures at end inspiration and expiration in volume-controlled ventilation and pressure-controlled ventilation modes in acute respiratory distress syndrome.
An active test lung (ASL 5000™) connected to five intensive care unit ventilators was used in a model of acute respiratory distress syndrome. Respiratory muscle efforts (muscle pressure) were configured in three different ways: no effort (muscle pressure: 0cmH2O); inspiratory efforts only (muscle pressure:-5cmH2O, neural inspiratory time of 0.6s); and both inspiratory and expiratory muscle efforts (muscle pressure:-5/+5cmH2O). Volume-controlled and pressure-controlled ventilation modes were set to deliver a target tidal volume of 420mL and positive end-expiratory pressure of 10cmH2O. The tidal volume delivered to the lungs, alveolar pressures at the end of inspiration, and alveolar pressures at end expiration were evaluated.
When triggered by the simulated patient, the median tidal volume was 27mL lower than the set tidal volume (range-63 to +79mL), and there was variation in alveolar pressures with a median of 25.4cmH2O (range 20.5 to 30cmH2O). In the simulated scenarios with both spontaneous inspiratory and expiratory muscle efforts and with a mandatory respiratory rate lower than the simulated patient's efforts, the median tidal volume was higher than controlled breathing.
Adjusting respiratory muscle effort and pulmonary ventilator respiratory rate to a value above the patient’s respiratory rate in assisted/controlled modes generated large variations in tidal volume and pulmonary pressures, while the controlled mode showed no variations in these outcomes.
Abstract
Rev Bras Ter Intensiva. 2021;33(3):461-468
DOI 10.5935/0103-507X.20210061
Spontaneous breathing can be deleterious in patients with previously injured lungs, especially in acute respiratory distress syndrome. Moreover, the failure to assume spontaneous breathing during mechanical ventilation and the need to switch back to controlled mechanical ventilation are associated with higher mortality. There is a gap of knowledge regarding which parameters might be useful to predict the risk of patient self-inflicted lung injury and to detect the inability to assume spontaneous breathing. We report a case of patient self-inflicted lung injury, the corresponding basic and advanced monitoring of the respiratory system mechanics and physiological and clinical results related to spontaneous breathing. The patient was a 33-year-old Caucasian man with a medical history of AIDS who developed acute respiratory distress syndrome and needed invasive mechanical ventilation after noninvasive ventilatory support failure. During the controlled ventilation periods, a protective ventilation strategy was adopted, and the patient showed clear clinical and radiographic improvement. However, during each spontaneous breathing period under pressure support ventilation, despite adequate initial parameters and a strictly adjusted ventilatory setting and monitoring, the patient developed progressive hypoxemia and worsening of respiratory system mechanics with a clearly correlated radiographic deterioration (patient self-inflicted lung injury). After failing three spontaneous breathing assumption trials, he died on day 29 due to refractory hypoxemia. Conventional basic and advanced monitoring variables in this case were not sufficient to identify the aptitude to breathe spontaneously or to predict the risk and development of patient self-inflicted lung injury during partial support ventilation.
Abstract
Rev Bras Ter Intensiva. 2020;32(1):58-65
DOI 10.5935/0103-507X.20200010
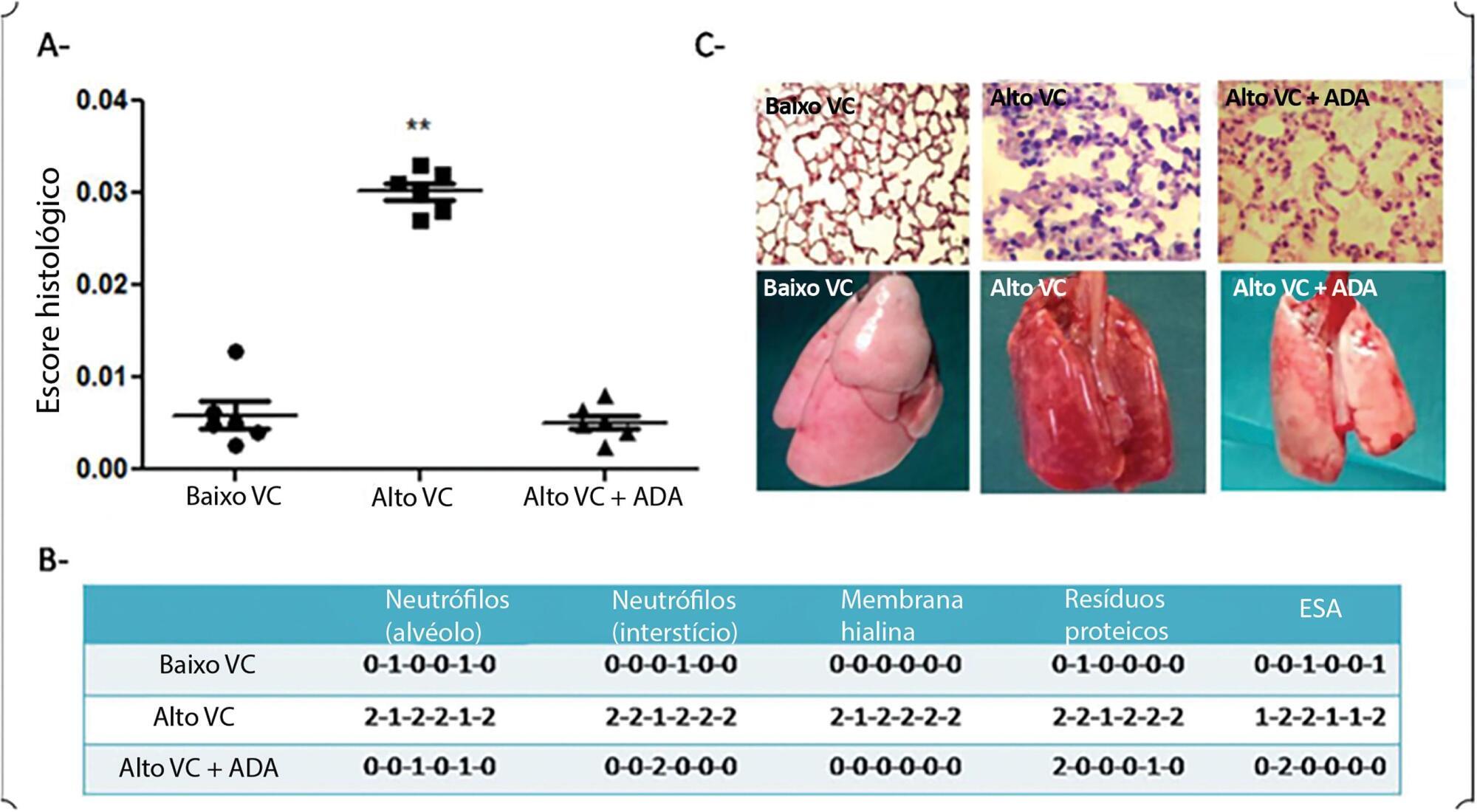
To determine whether adalimumab administration before mechanical ventilation reduces ventilator-induced lung injury (VILI).
Eighteen rats randomized into 3 groups underwent mechanical ventilation for 3 hours with a fraction of inspired oxygen = 0.40% including a low tidal volume group (n = 6), where tidal volume = 8mL/kg and positive end-expiratory pressure = 5cmH2O; a high tidal volume group (n = 6), where tidal volume = 35mL/kg and positive end-expiratory pressure = 0; and a pretreated + high tidal volume group (n = 6) where adalimumab (100ug/kg) was administered intraperitoneally 24 hours before mechanical ventilation + tidal volume = 35mL/kg and positive end-expiratory pressure = 0. ANOVA was used to compare histological damage (ATS 2010 Lung Injury Scoring System), pulmonary edema, lung compliance, arterial partial pressure of oxygen, and mean arterial pressure among the groups.
After 3 hours of ventilation, the mean histological lung injury score was higher in the high tidal volume group than in the low tidal volume group (0.030 versus 0.0051, respectively, p = 0.003). The high tidal volume group showed diminished lung compliance at 3 hours (p = 0.04) and hypoxemia (p = 0,018 versus control). Pretreated HVt group had an improved histological score, mainly due to a significant reduction in leukocyte infiltration (p = 0.003).
Histological examination after 3 hours of injurious ventilation revealed ventilator-induced lung injury in the absence of measurable changes in lung mechanics or oxygenation; administering adalimumab before mechanical ventilation reduced lung edema and histological damage.
Abstract
Rev Bras Ter Intensiva. 2018;30(2):208-218
DOI 10.5935/0103-507X.20180038
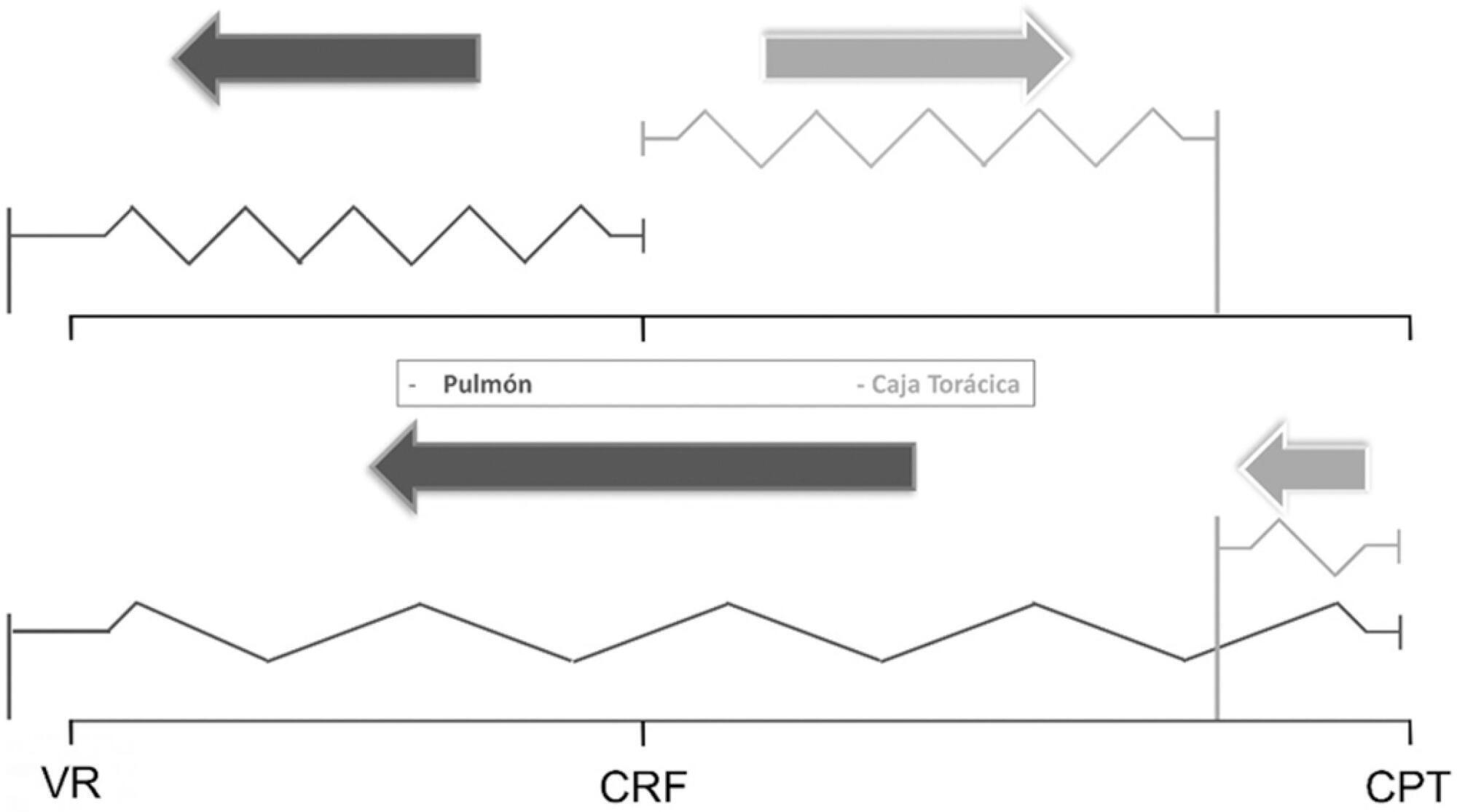
The respiratory system mechanics depend on the characteristics of the lung and chest wall and their interaction. In patients with acute respiratory distress syndrome under mechanical ventilation, the monitoring of airway plateau pressure is fundamental given its prognostic value and its capacity to assess pulmonary stress. However, its validity can be affected by changes in mechanical characteristics of the chest wall, and it provides no data to correctly titrate positive end-expiratory pressure by restoring lung volume. The chest wall effect on respiratory mechanics in acute respiratory distress syndrome has not been completely described, and it has likely been overestimated, which may lead to erroneous decision making. The load imposed by the chest wall is negligible when the respiratory system is insufflated with positive end-expiratory pressure. Under dynamic conditions, moving this structure demands a pressure change whose magnitude is related to its mechanical characteristics, and this load remains constant regardless of the volume from which it is insufflated. Thus, changes in airway pressure reflect changes in the lung mechanical conditions. Advanced monitoring could be reserved for patients with increased intra-abdominal pressure in whom a protective mechanical ventilation strategy cannot be implemented. The estimates of alveolar recruitment based on respiratory system mechanics could reflect differences in chest wall response to insufflation and not actual alveolar recruitment.
Abstract
Rev Bras Ter Intensiva. 2017;29(2):231-237
DOI 10.5935/0103-507X.20170032
Overdistention and intratidal alveolar recruitment have been advocated as the main physical mechanisms responsible for ventilator-induced lung injury. Limiting tidal volume has a demonstrated survival benefit in patients with acute respiratory distress syndrome and is recognized as the cornerstone of protective ventilation. In contrast, the use of high positive end-expiratory pressure levels in clinical trials has yielded conflicting results and remains controversial. In the present review, we will discuss the benefits and limitations of the open lung approach and will discuss some recent experimental and clinical trials on the use of high versus low/moderate positive end-expiratory pressure levels. We will also distinguish dynamic (tidal volume) from static strain (positive end-expiratory pressure and mean airway pressure) and will discuss their roles in inducing ventilator-induced lung injury. High positive end-expiratory pressure strategies clearly decrease refractory hypoxemia in patients with acute respiratory distress syndrome, but they also increase static strain, which in turn may harm patients, especially those with lower levels of lung recruitability. In patients with severe respiratory failure, titrating positive end-expiratory pressure against the severity of hypoxemia, or providing it in a decremental fashion after a recruitment maneuver, is recommended. If high plateau, driving or mean airway pressures are observed, prone positioning or ultraprotective ventilation may be indicated to improve oxygenation without additional stress and strain in the lung.
Abstract
Rev Bras Ter Intensiva. 2013;25(4):319-326
DOI 10.5935/0103-507X.20130054
In preterm infants, the need for intubation and mechanical ventilation is associated with ventilator-induced lung injuries and subsequent bronchopulmonary dysplasia. The aim of the present review was to improve the understanding of the mechanisms of injury that involve cytokine-mediated inflammation to contribute to the development of new preventive strategies. Relevant articles were retrieved from the PubMed database using the search terms "ventilator-induced lung injury preterm", "continuous positive airway pressure", "preterm", and "bronchopulmonary dysplasia". The resulting data and other relevant information were divided into several topics to ensure a thorough, critical view of ventilation-induced lung injury and its consequences in preterm infants. The role of pro-inflammatory cytokines (particularly interleukins 6 and 8 and tumor necrosis factor alpha) as mediators of lung injury was assessed. Evidence from studies conducted with animals and human newborns is described. This evidence shows that brief periods of mechanical ventilation is sufficient to induce the release of pro-inflammatory cytokines. Other forms of mechanical and non-invasive ventilation were also analyzed as protective alternatives to conventional mechanical ventilation. It was concluded that non-invasive ventilation, intubation followed by early surfactant administration and quick extubation for nasal continuous positive airway pressure, and strategies that regulate tidal volume and avoid volutrauma (such as volume guarantee ventilation) protect against ventilator-induced lung injury in preterm infants.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)