Abstract
Crit Care Sci. 2024;36:e20240235en
DOI 10.62675/2965-2774.20240235-pt
Newborn infants admitted to the neonatal intensive care unit require arterial cannulation for hemodynamic monitoring and blood sampling. Arterial access is achieved through catheterization of umbilical or peripheral arteries. Peripheral artery cannulation is performed in critically ill newborns, but artery localization and cannulation is often challenging and unsuccessful. Therefore, increasing the internal diameter and preventing vasospasm are important for successful peripheral artery cannulation in neonates. Topical glyceryl trinitrate has the potential to increase cannulation success by relaxing arterial smooth muscles and thus increasing the internal diameter. We aim to conduct a pilot randomized controlled trial to evaluate the efficacy and safety of topycal glyceryl trinitrate in increasing the diameter of the radial artery in neonates.
This study will be a single-center, observer-blind, randomized, placebo-controlled trial conducted in the neonatal intensive care unit of Perth Children's Hospital, Western Australia. A total of 60 infants born at >34 weeks of gestation who are admitted for elective surgery or medical reasons and for whom a peripheral arterial line is needed for sampling or blood pressure monitoring will be recruited after informed parental consent is obtained. The primary outcome will be the change in radial arterial diameter from baseline to postintervention. Secondary outcomes will be the absolute and percentage change from baseline in the radial arterial diameter in both limbs and safety (hypotension and methemoglobinemia).
This will be the first randomized controlled trial evaluating the use of topical glyceryl trinitrate to facilitate peripheral artery cannulation in neonates. If our pilot randomized controlled trial confirms the benefits of glyceryl trinitrate patches, it will pave the way for large multicenter randomized controlled trials in this field.
Abstract
Rev Bras Ter Intensiva. 2021;33(3):434-439
DOI 10.5935/0103-507X.20210058
To evaluate pain intensity during arterial puncture performed in newborns admitted to a neonatal progressive care unit and to evaluate the perception of health professionals regarding neonatal pain.
This was an observational analytical study in which 62 arterial punctures were performed in 35 neonates. Pain was assessed during collection using the Premature Infant Pain Profile scale. The health professionals responsible for collection evaluated pain using a verbal numerical scale ranging from zero to ten. The data were subjected to descriptive statistical analysis using the Statistical Package for the Social Science software.
Among the newborns, 30.6% (n = 19) had no pain or mild pain (0 - 6), 24.2% (n = 15) had mild to moderate pain (7 - 11) and 45.2% (28) had severe pain (12 - 21). It was found that health professionals identified pain during the procedure.
Arterial puncture is considered a painful procedure that can result in mild to severe pain. The adoption of systematic evaluation strategies is necessary to enable appropriate therapeutic intervention.
Abstract
Rev Bras Ter Intensiva. 2021;33(2):266-275
DOI 10.5935/0103-507X.20210034
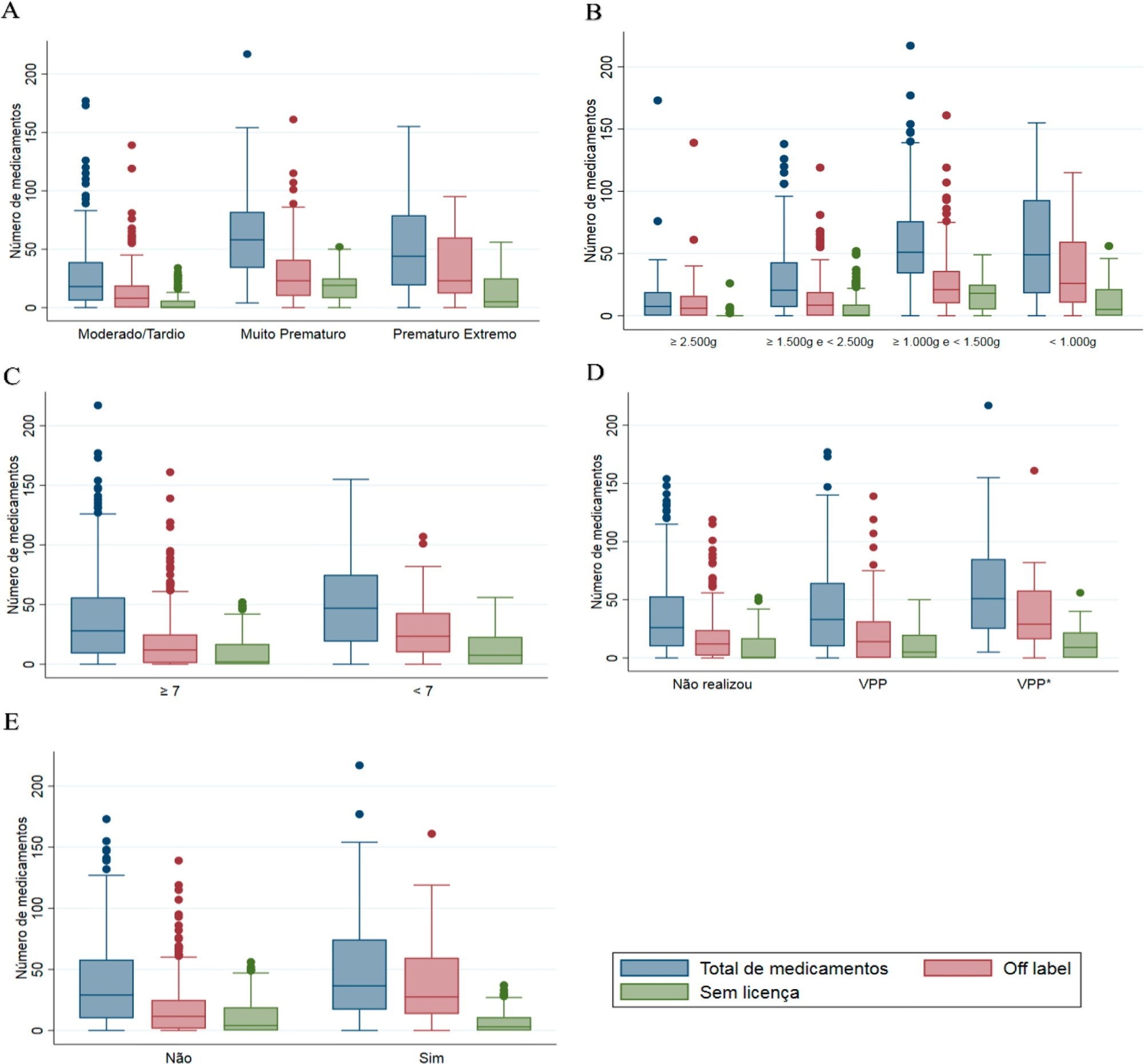
To evaluate the use of off-label and unlicensed medications in preterm infants hospitalized in a neonatal intensive care unit.
This nonconcurrent cohort study included preterm infants admitted to 3 neonatal intensive care units in 2016 and 2017 who were followed up during the neonatal period. The type and number of medications used were recorded for the entire period and classified based on the Anatomical Therapeutic Chemical. Descriptive and bivariate data analyses were performed to assess associations between the number of drugs used (total, off-label and unlicensed) and the explanatory variables of interest.
Four hundred preterm infants received 16,143 prescriptions for 86 different pharmaceuticals; 51.9% of these medications were classified as off-label and 23.5% as unlicensed. The most prescribed drugs were gentamicin and ampicillin (17.5% and 15.5% among off-label, respectively) and caffeine (75.5% among unlicensed). The results indicated significant associations between the use of off-label drugs and lower gestational age, low birth weight, lower 5-minute Apgar score, advanced resuscitation maneuver in the delivery room and death. The prescription of unlicensed drugs was associated with lower gestational age, low birth weight and 5-minute Apgar score below 7.
Neonates admitted to neonatal intensive care units are highly exposed to off-label and unlicensed medications. Further studies are needed to achieve greater safety and quality of drug therapy used in neonatology.
Abstract
Rev Bras Ter Intensiva. 2021;33(1):12-30
DOI 10.5935/0103-507X.20210002
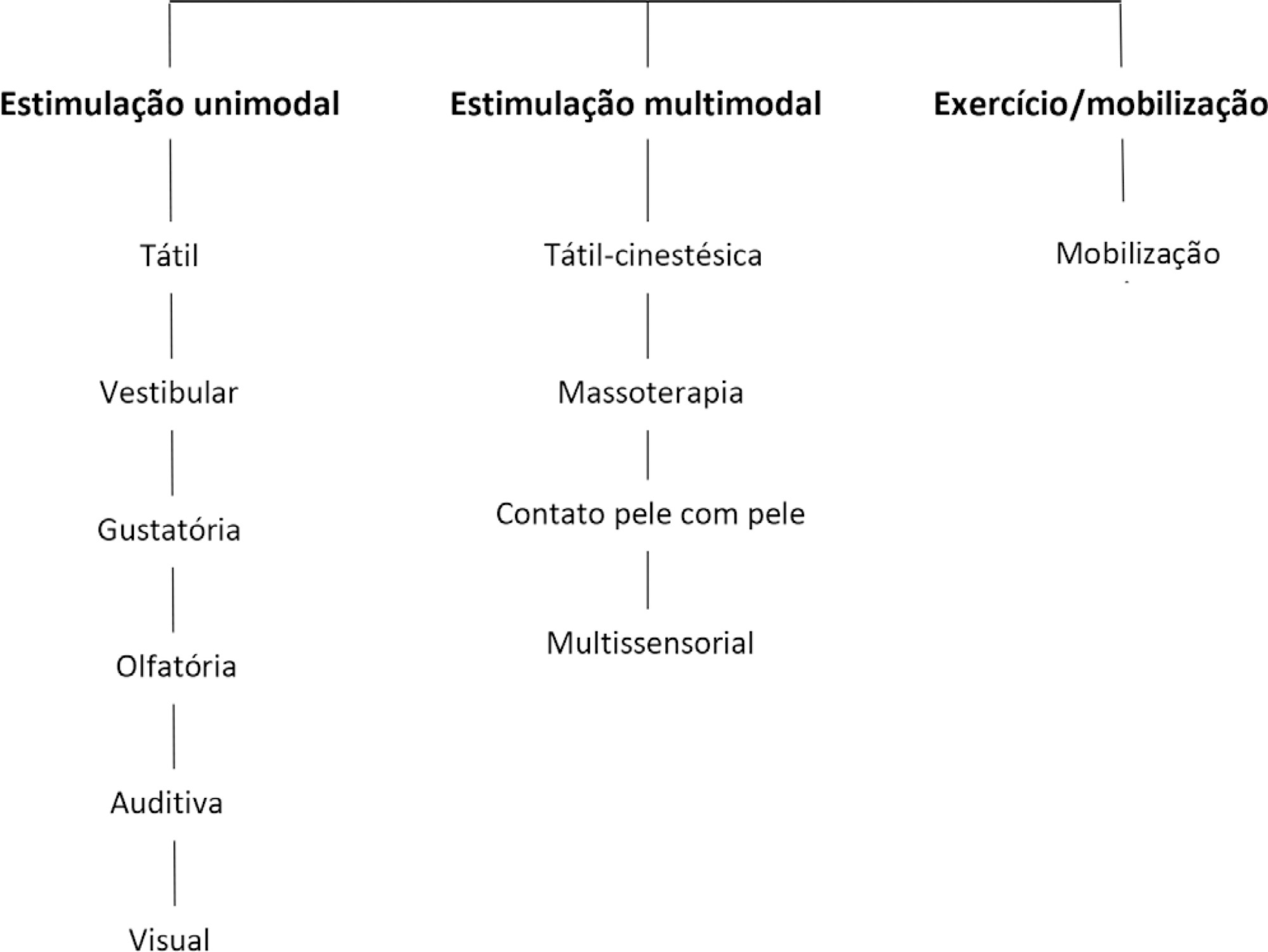
To present guidelines on sensory motor stimulation for newborns and infants in the intensive care unit.
We employed a mixed methods design with a systematic review of the literature and recommendations based on scientific evidence and the opinions of physiotherapists with neonatal expertise. The research included studies published between 2010 and 2018 in the MEDLINE® and Cochrane databases that included newborns (preterm and term) and infants (between 28 days and 6 months of age) hospitalized in the intensive care unit and submitted to sensory motor stimulation methods. The studies found were classified according to the GRADE score by five physiotherapists in different regions of Brazil and presented at eight Scientific Congresses held to discuss the clinical practice guidelines.
We included 89 articles to construct the clinical practice guidelines. Auditory, gustatory and skin-to-skin stimulation stand out for enhancing vital signs, and tactile-kinesthetic massage and multisensory stimulation stand out for improving weight or sucking.
Although all modalities have good ratings for pain or stress control, it is recommended that sensory motor stimulation procedures be tailored to the infant’s specific needs and that interventions and be carried out by expert professionals.
Abstract
Rev Bras Ter Intensiva. 2020;32(2):235-243
DOI 10.5935/0103-507X.20200038
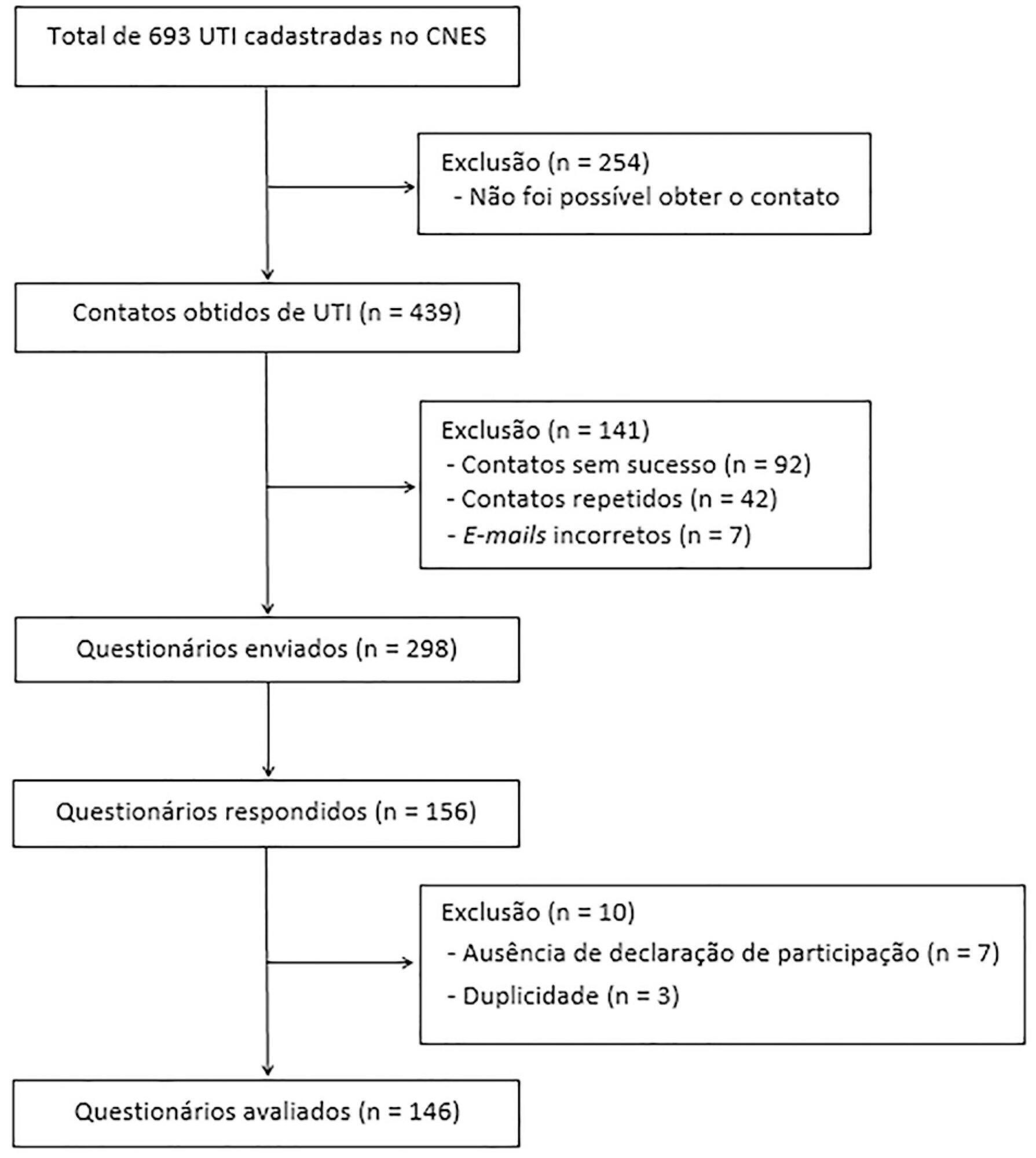
To identify the neonatal, pediatric and mixed (neonatal and pediatric) intensive care units in Brazil that use cuffed tracheal tubes in clinical practice and to describe the characteristics related to the use of protocols and monitoring.
To identify the intensive care units in Brazil, the Ministry of Health’s National Registry of Health Facilities was accessed, and information was collected on 693 registered intensive care units. This was an analytical cross-sectional survey conducted through electronic questionnaires sent to 298 neonatal, pediatric and mixed intensive care units in Brazil.
This study analyzed 146 questionnaires (49.3% from neonatal intensive care units, 35.6% from pediatric intensive care units and 15.1% from mixed pediatric intensive care units). Most of the participating units (78/146) used cuffed tracheal tubes, with a predominance of use in pediatric intensive care units (52/78). Most of the units that used cuffed tracheal tubes applied a cuff pressure monitoring protocol (45/78). The use of cuff monitoring protocols was observed in intensive care units with a physical therapy service exclusive to the unit (38/61) and in those with a physical therapist present 24 hours/day (25/45). The most frequent cause of extubation failure related to the use of cuffed tracheal tubes in pediatric intensive care units was upper airway obstruction.
In this survey, the use of cuffed tracheal tubes and the application of a cuff pressure monitoring protocol was predominant in pediatric intensive care units. The use of a monitoring protocol was more common in intensive care units that had a physical therapist who was exclusive to the unit and was present 24 hours/day.
Abstract
Rev Bras Ter Intensiva. 2019;31(3):347-353
DOI 10.5935/0103-507X.20190047
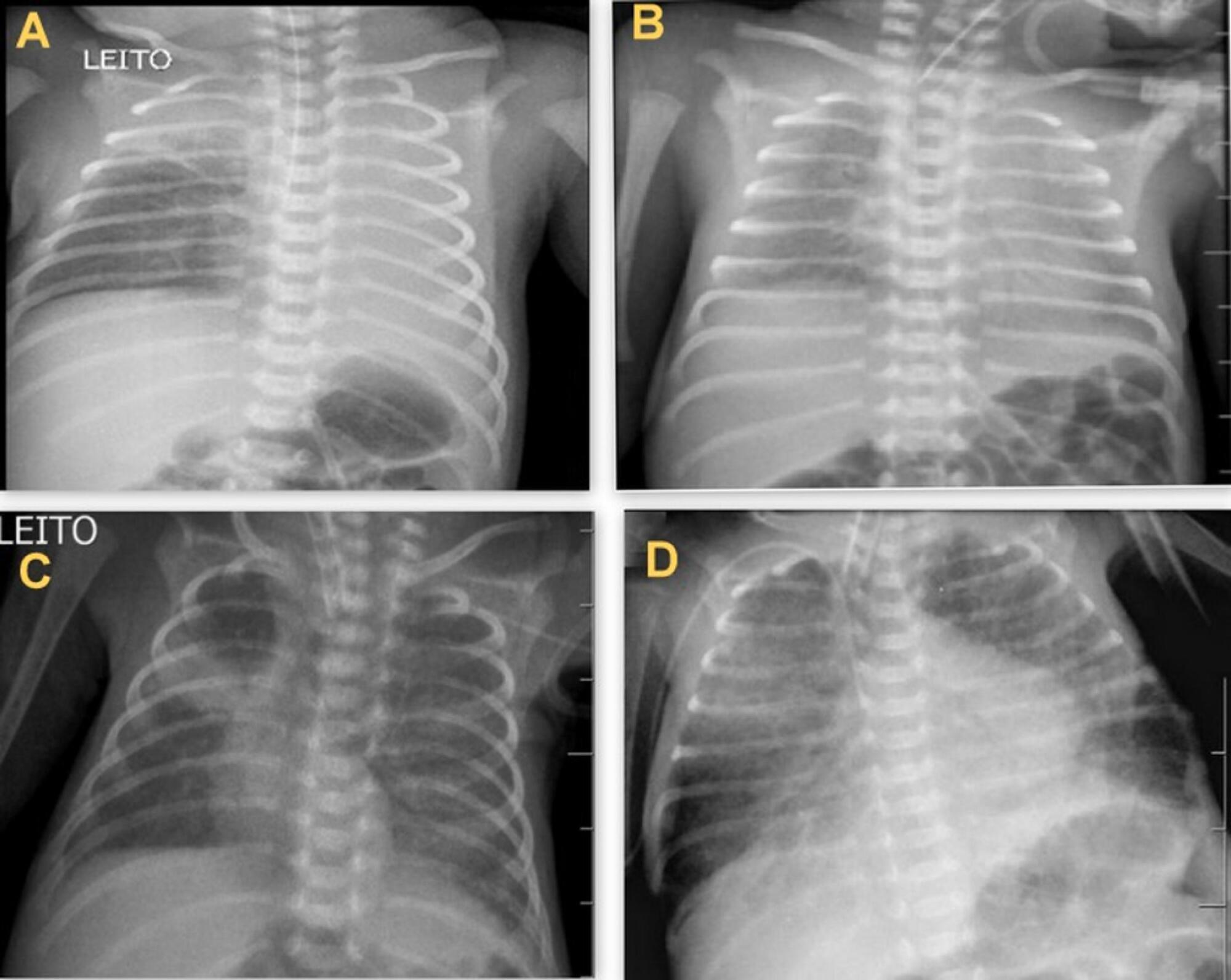
To determine the occurrence and characteristics of atelectasis, opacities, hypolucency and pulmonary infiltrates observed on chest X-rays of preterm infants in a neonatal intensive care unit.
This was a cross-sectional observational study. From August to December 2017, all chest radiographs of newborn infants were analyzed. The study included the chest radiographs of preterm neonates with gestational ages up to 36 weeks in the neonatal period that showed clear changes or suspected changes, which were confirmed after a radiologist’s report. Radiological changes were associated with possible predisposing factors.
During the study period, 450 radiographs were performed on preterm neonates, and 37 lung changes were identified and classified into 4 types: 12 (2.66%) changes were described as opacities, 11 (2.44%) were described as atelectasis, 10 (2.22%) were described as pulmonary infiltrate, and 4 (0.88%) were described as hypolucency. A higher occurrence of atelectasis was noted in the right lung (81.8%). Among the abnormal radiographs, 25 (67.6%) newborn infants were receiving invasive mechanical ventilation.
Considering the radiological report, no significance was found for the observed changes. Atelectasis was not the most frequently observed change. The predisposing factors for these changes were extreme prematurity, low weight, male sex, a poorly positioned endotracheal tube and the use of invasive mechanical ventilation.
Abstract
Rev Bras Ter Intensiva. 2019;31(1):21-26
DOI 10.5935/0103-507X.20190007
This study sought to describe and quantify the pharmacological and nonpharmacological strategies used to relieve the pain/stress of neonates during hospitalization in neonatal intensive care units.
This quantitative, longitudinal, and descriptive study examined 50 neonates from neonatal intensive care unit admission to discharge.
A total of 9,948 painful/stressful procedures were recorded (mean = 11.25 ± 6.3) per day per neonate. A total of 11,722 pain-management and relief interventions were performed, of which 11,495 (98.1%) were nonpharmacological strategies, and 227 (1.9%) were pharmacological interventions. On average, each neonate received 235 pain-management and treatment interventions during hospitalization, 13 nonpharmacological interventions per day, and one pharmacological intervention every 2 days.
Neonates receive few specific measures for pain relief given the high number of painful and stressful procedures performed during hospitalization. Thus, it is essential to implement effective pain-relief protocols.
Abstract
Rev Bras Ter Intensiva. 2018;30(2):195-200
DOI 10.5935/0103-507X.20180033
This study sought to translate the Cornell Assessment of Pediatric Delirium from English into Brazilian Portuguese and cross-culturally adapt it for use in Brazil.
Following the authorization granted by its main author, the processes of translation and cross-cultural adaptation were performed with regard to the Cornell Assessment of Pediatric Delirium in accordance with the following internationally recommended steps: translation of the original into Portuguese by two native speakers of the target language; synthesis of the translated versions; back-translation by two native speakers of the original language; review and harmonization of the back-translation; a review of the Portuguese version of the Cornell Assessment of Pediatric Delirium by an expert panel composed of specialists; pretesting including assessments of clarity, comprehensibility, and acceptability of the translated version using a sample of the target population; and finishing modifications to achieve the final version.
The translation and cross-cultural adaptation of the Cornell Assessment of Pediatric Delirium followed international recommendations. The linguistic and semantic issues that emerged during the process were discussed by the expert panel, which unanimously agreed to slight modifications. During pretesting, the Cornell Assessment of Pediatric Delirium was administered to 30 eligible children, twice per day; the final version was easy to understand, could be completed quickly, and showed a high inter-rater correlation coefficient (0.955).
The translation of the Cornell Assessment of Pediatric Delirium into Brazilian Portuguese and its cross-cultural adaptation were successful and preserved the linguistic and semantic properties of the original instrument. The Cornell Assessment of Pediatric Delirium proved to be easy to understand and could be completed quickly. Additional studies are needed to test the validity and psychometric properties of this version in Brazil.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (33) COVID-19 (45) Critical care (115) Critical illness (54) ICU (25) Infant, newborn (27) Intensive care (72) Intensive care units (254) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (75) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (117) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)