Abstract
Rev Bras Ter Intensiva. 2021;33(2):266-275
DOI 10.5935/0103-507X.20210034
To evaluate the use of off-label and unlicensed medications in preterm infants hospitalized in a neonatal intensive care unit.
This nonconcurrent cohort study included preterm infants admitted to 3 neonatal intensive care units in 2016 and 2017 who were followed up during the neonatal period. The type and number of medications used were recorded for the entire period and classified based on the Anatomical Therapeutic Chemical. Descriptive and bivariate data analyses were performed to assess associations between the number of drugs used (total, off-label and unlicensed) and the explanatory variables of interest.
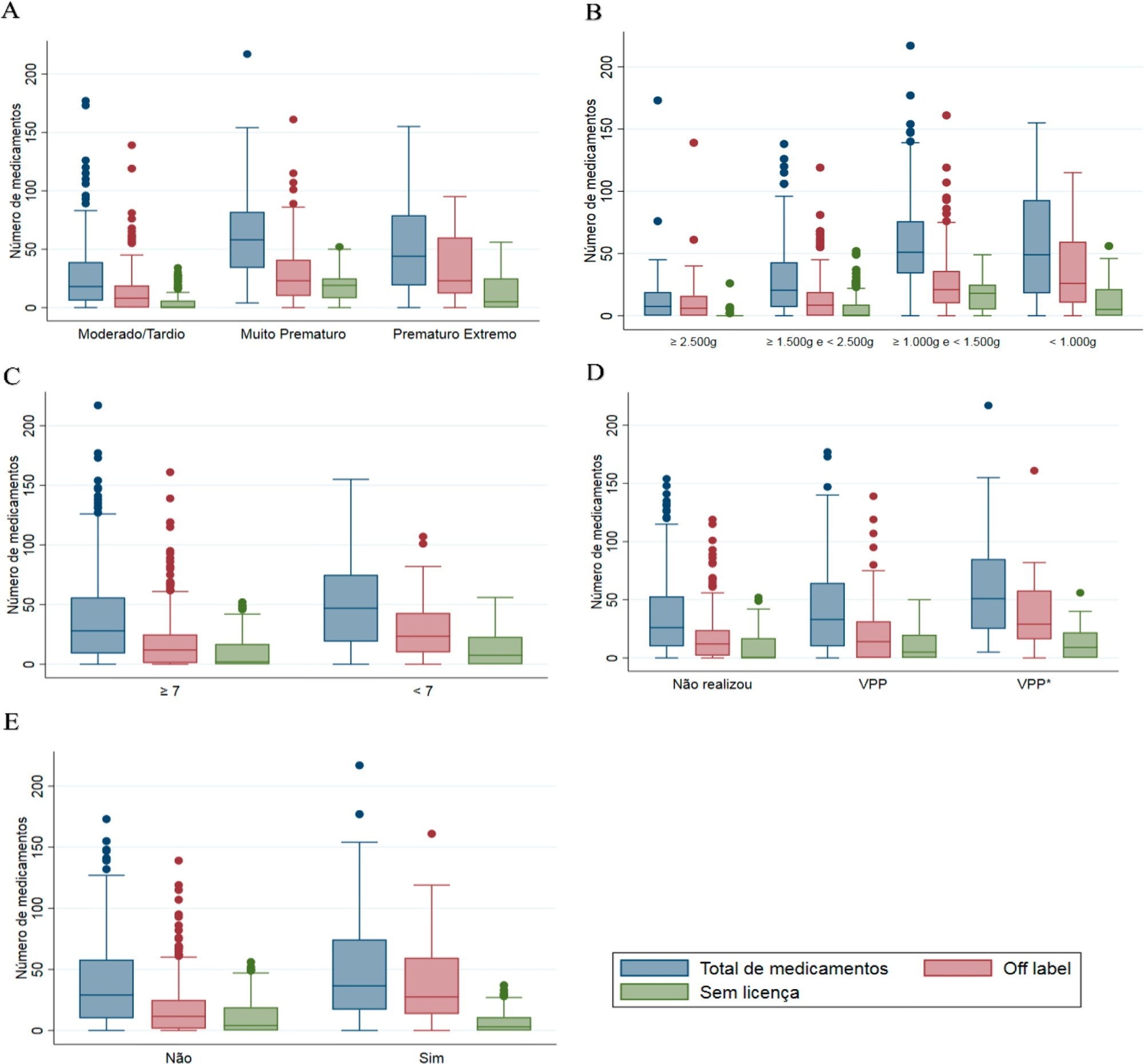
Four hundred preterm infants received 16,143 prescriptions for 86 different pharmaceuticals; 51.9% of these medications were classified as off-label and 23.5% as unlicensed. The most prescribed drugs were gentamicin and ampicillin (17.5% and 15.5% among off-label, respectively) and caffeine (75.5% among unlicensed). The results indicated significant associations between the use of off-label drugs and lower gestational age, low birth weight, lower 5-minute Apgar score, advanced resuscitation maneuver in the delivery room and death. The prescription of unlicensed drugs was associated with lower gestational age, low birth weight and 5-minute Apgar score below 7.
Neonates admitted to neonatal intensive care units are highly exposed to off-label and unlicensed medications. Further studies are needed to achieve greater safety and quality of drug therapy used in neonatology.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)