Abstract
Rev Bras Ter Intensiva. 2020;32(2):235-243
DOI 10.5935/0103-507X.20200038
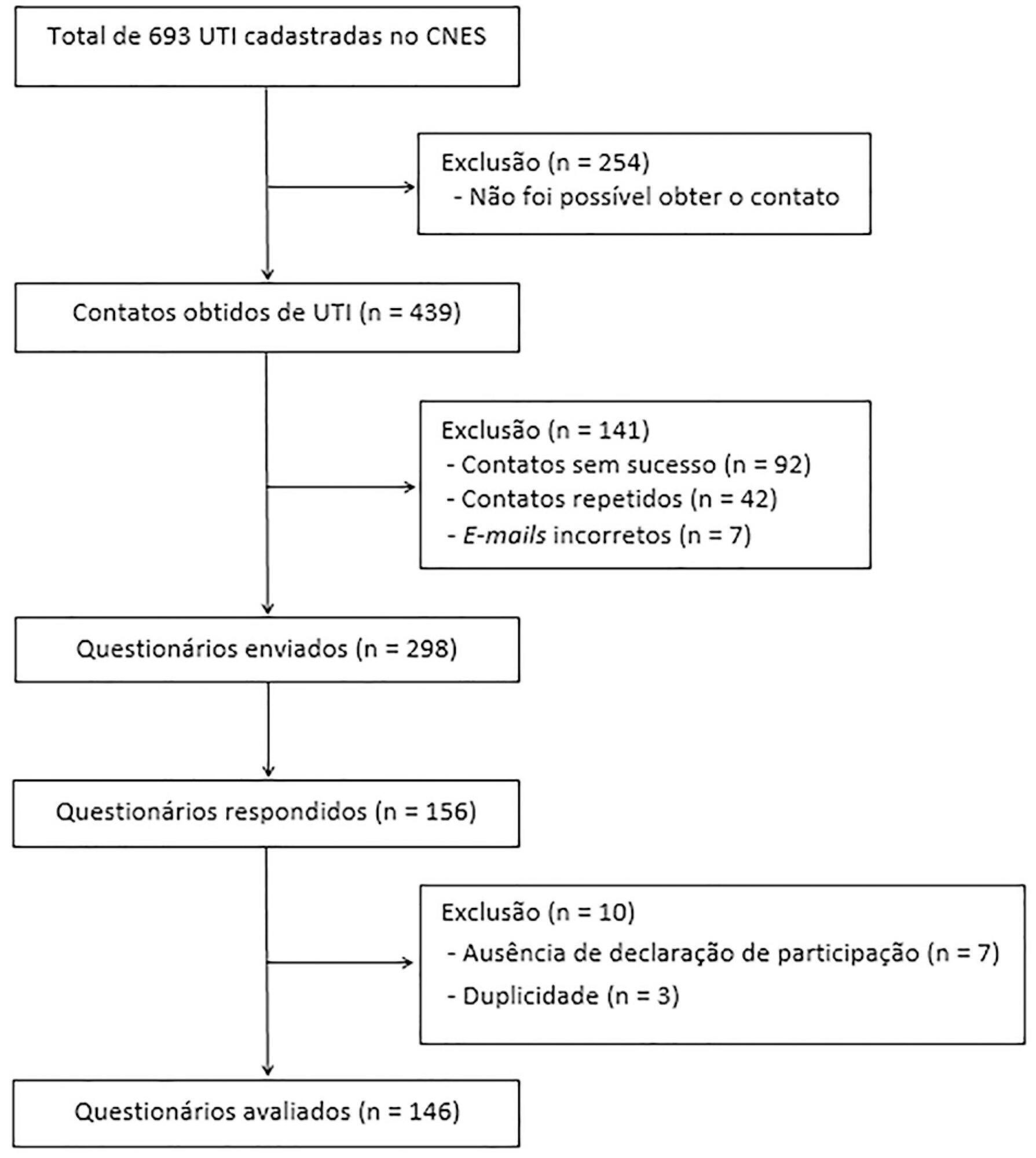
To identify the neonatal, pediatric and mixed (neonatal and pediatric) intensive care units in Brazil that use cuffed tracheal tubes in clinical practice and to describe the characteristics related to the use of protocols and monitoring.
To identify the intensive care units in Brazil, the Ministry of Health’s National Registry of Health Facilities was accessed, and information was collected on 693 registered intensive care units. This was an analytical cross-sectional survey conducted through electronic questionnaires sent to 298 neonatal, pediatric and mixed intensive care units in Brazil.
This study analyzed 146 questionnaires (49.3% from neonatal intensive care units, 35.6% from pediatric intensive care units and 15.1% from mixed pediatric intensive care units). Most of the participating units (78/146) used cuffed tracheal tubes, with a predominance of use in pediatric intensive care units (52/78). Most of the units that used cuffed tracheal tubes applied a cuff pressure monitoring protocol (45/78). The use of cuff monitoring protocols was observed in intensive care units with a physical therapy service exclusive to the unit (38/61) and in those with a physical therapist present 24 hours/day (25/45). The most frequent cause of extubation failure related to the use of cuffed tracheal tubes in pediatric intensive care units was upper airway obstruction.
In this survey, the use of cuffed tracheal tubes and the application of a cuff pressure monitoring protocol was predominant in pediatric intensive care units. The use of a monitoring protocol was more common in intensive care units that had a physical therapist who was exclusive to the unit and was present 24 hours/day.
Abstract
Rev Bras Ter Intensiva. 2017;29(1):55-62
DOI 10.5935/0103-507X.20170009
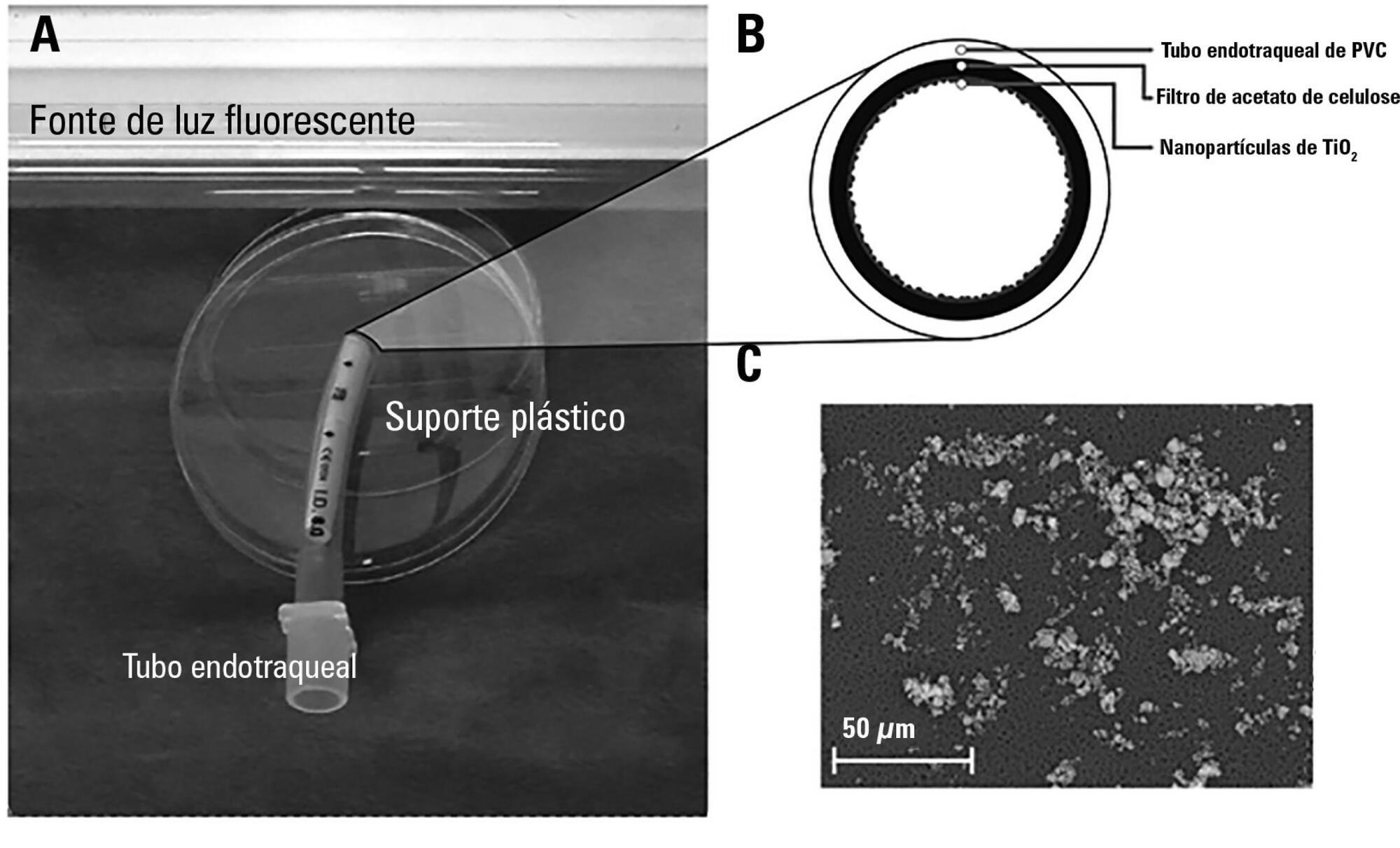
The aim of this study was to assess the antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa of two nanoparticle endotracheal tube coatings with visible light-induced photocatalysis.
Two types of titanium dioxide nanoparticles were tested: standard anatase (TiO2) and N-doped TiO2 (N-TiO2). Nanoparticles were placed on the internal surface of a segment of commercial endotracheal tubes, which were loaded on a cellulose acetate filter; control endotracheal tubes were left without a nanoparticle coating. A bacterial inoculum of 150 colony forming units was placed in the endotracheal tubes and then exposed to a fluorescent light source (3700 lux, 300-700 nm wavelength) for 5, 10, 20, 40, 60 and 80 minutes. Colony forming units were counted after 24 hours of incubation at 37°C. Bacterial inactivation was calculated as the percentage reduction of bacterial growth compared to endotracheal tubes not exposed to light.
In the absence of light, no relevant antibacterial activity was shown against neither strain. For P. aeruginosa, both coatings had a higher bacterial inactivation than controls at any time point (p < 0.001), and no difference was observed between TiO2 and N-TiO2. For S. aureus, inactivation was higher than for controls starting at 5 minutes for N-TiO2 (p = 0.018) and 10 minutes for TiO2 (p = 0.014); inactivation with N-TiO2 was higher than that with TiO2 at 20 minutes (p < 0.001), 40 minutes (p < 0.001) and 60 minutes (p < 0.001).
Nanosized commercial and N-doped TiO2 inhibit bacterial growth under visible fluorescent light. N-TiO2 has higher antibacterial activity against S. aureus compared to TiO2.
Abstract
Rev Bras Ter Intensiva. 2016;28(3):330-334
DOI 10.5935/0103-507X.20160056
To identify and evaluate the correct positioning of the most commonly used medical devices as visualized in thoracic radiograms of patients in the intensive care unit of our center.
A literature search was conducted for the criteria used to evaluate the correct positioning of medical devices on thoracic radiograms. All the thoracic radiograms performed in the intensive care unit of our center over an 18-month period were analyzed. All admissions in which at least one thoracic radiogram was performed in the intensive care unit and in which at least one medical device was identifiable in the thoracic radiogram were included. One radiogram per admission was selected for analysis. The radiograms were evaluated by an independent observer.
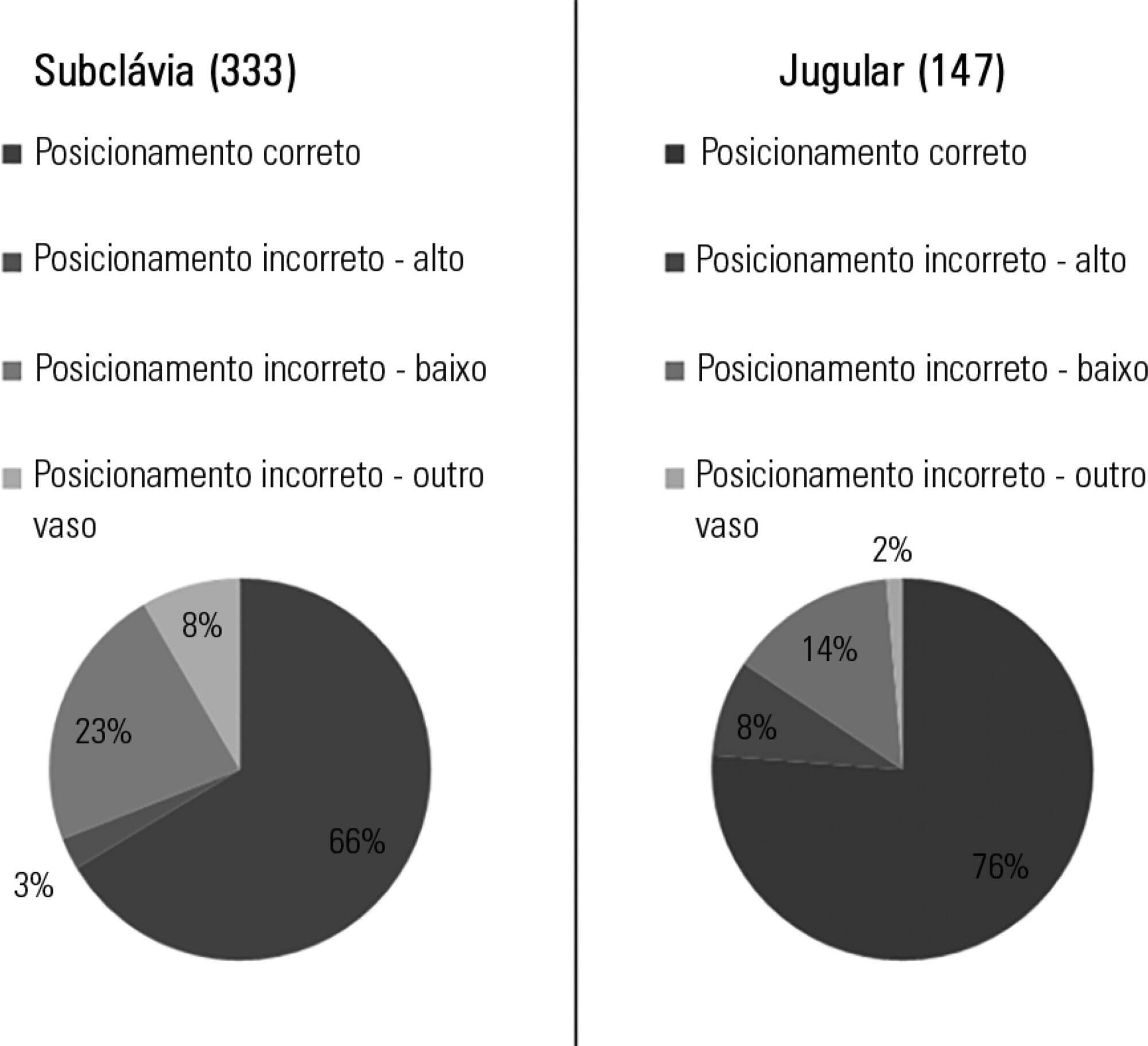
Out of the 2,312 thoracic radiograms analyzed, 568 were included in this study. Several medical devices were identified, including monitoring leads, endotracheal and tracheostomy tubes, central venous catheters, pacemakers and prosthetic cardiac valves. Of the central venous catheters that were identified, 33.6% of the subclavian and 23.8% of the jugular were malpositioned. Of the endotracheal tubes, 19.9% were malpositioned, while all the tracheostomy tubes were correctly positioned.
Malpositioning of central venous catheters and endotracheal tubes is frequently identified in radiograms of patients in an intensive care unit. This is relevant because malpositioned devices may be related to adverse events. In future studies, an association between malpositioning and adverse events should be investigated.
Abstract
Rev Bras Ter Intensiva. 2015;27(3):228-234
DOI 10.5935/0103-507X.20150037
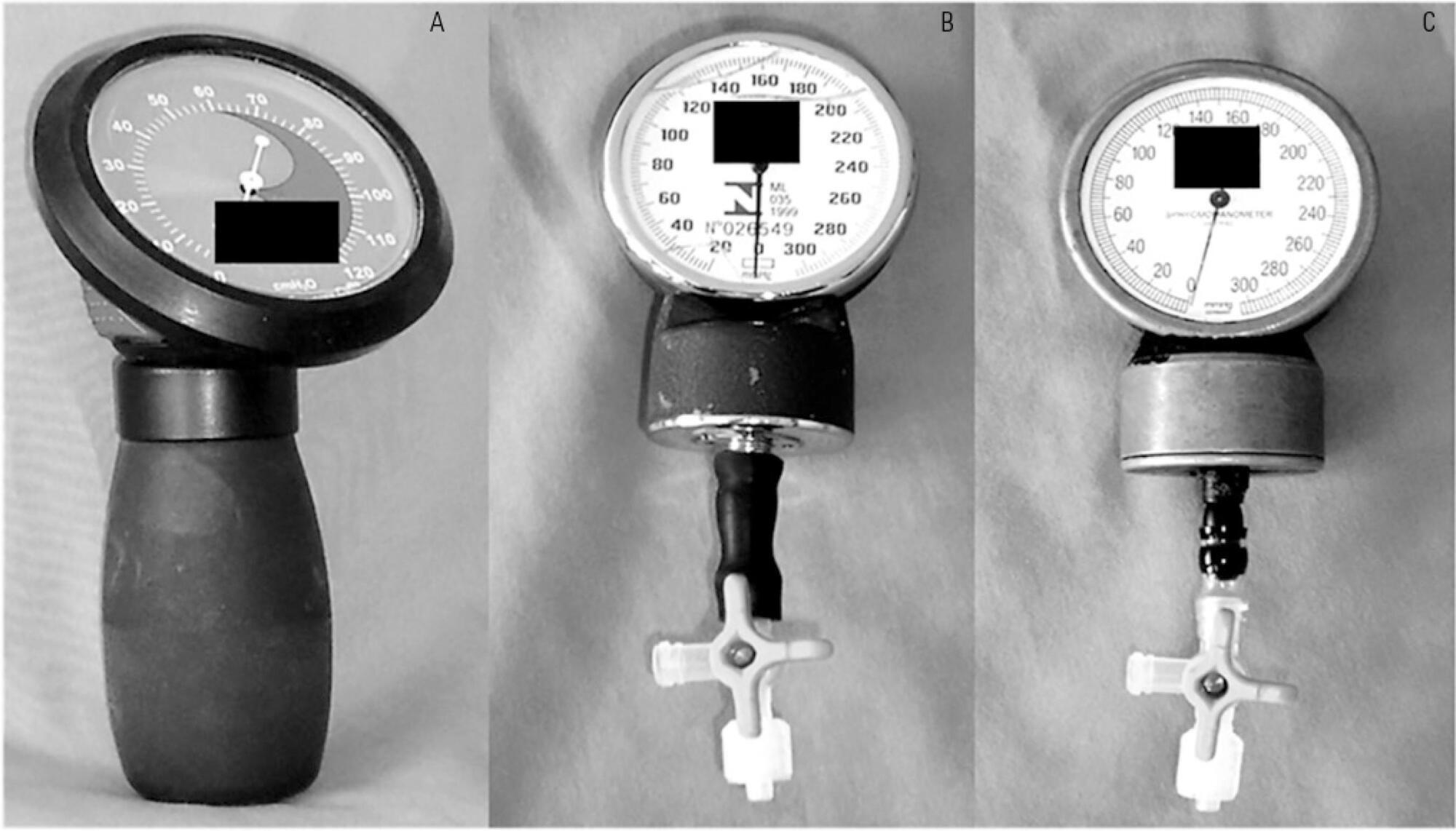
To test the agreement between two handcrafted devices and a cuff-specific manometer.
The agreement between two handcrafted devices adapted to measure tracheal tube cuff pressure and a cuff-specific manometer was tested on 79 subjects. The cuff pressure was measured with a commercial manometer and with two handcrafted devices (HD) assembled with aneroid sphygmomanometers (HD1 and HD2). The data were compared using Wilcoxon and Spearman tests, the intraclass correlation coefficient (ICC) and limit-of-agreement analysis.
Cuff pressures assessed with handcrafted devices were significantly different from commercial device measurements (pressures were higher when measured with HD1 and lower with HD2). The ICCs between the commercial device and HD1 and HD2 were excellent (ICC = 0.8 p < 0.001) and good (ICC = 0.66, p < 0.001), respectively. However, the Bland- Altman plots showed wide limits of agreement between HD1 and HD2 and the commercial device.
The handcrafted manometers do not provide accurate cuff pressure measurements when compared to a cuff-specific device and should not be used to replace the commercial cuff manometers in mechanically ventilated patients.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (115) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)