Abstract
Rev Bras Ter Intensiva. 2017;29(2):142-153
DOI 10.5935/0103-507X.20170024
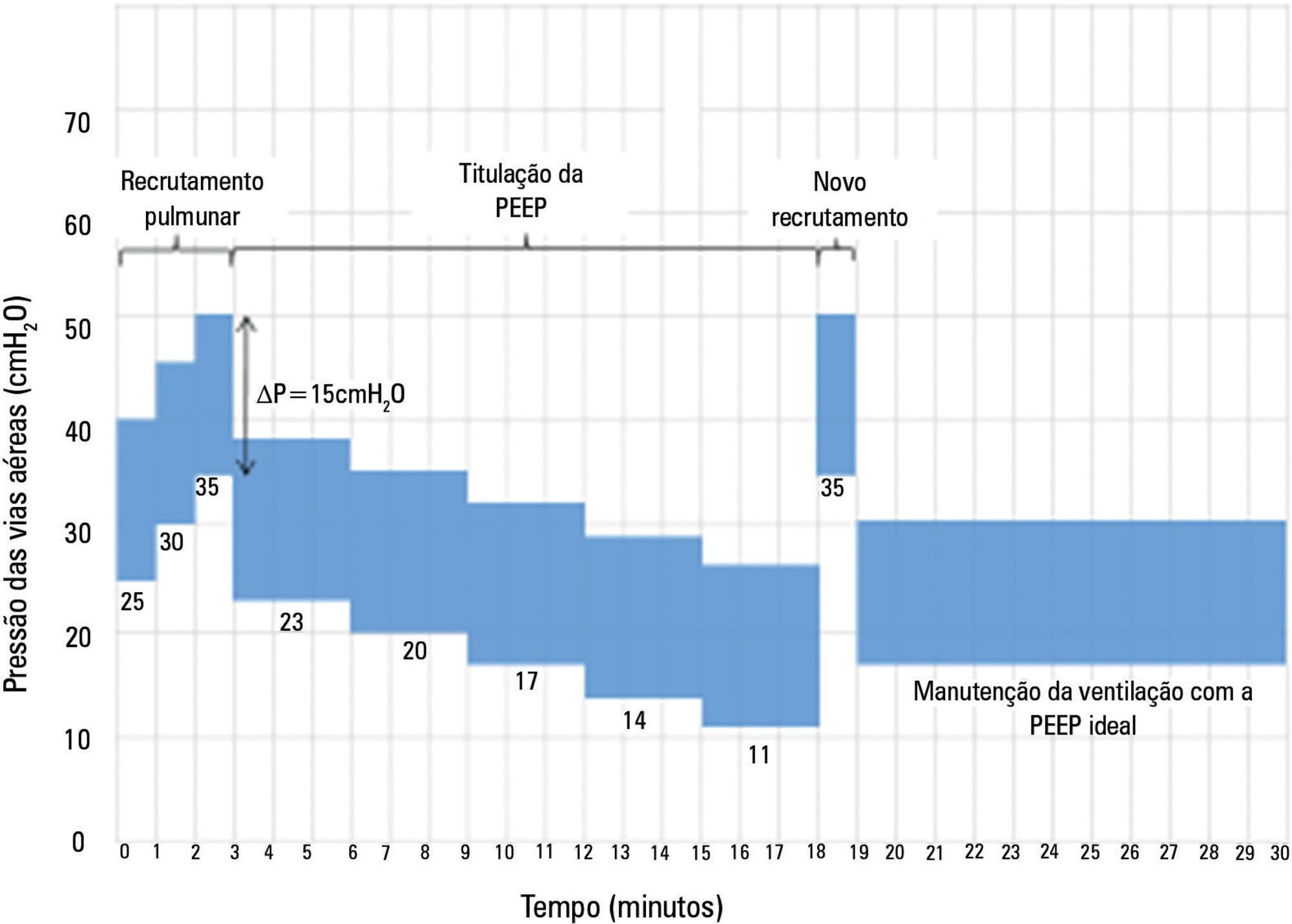
The Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) is an international multicenter randomized pragmatic controlled trial with allocation concealment involving 120 intensive care units in Brazil, Argentina, Colombia, Italy, Poland, Portugal, Malaysia, Spain, and Uruguay. The primary objective of ART is to determine whether maximum stepwise alveolar recruitment associated with PEEP titration, adjusted according to the static compliance of the respiratory system (ART strategy), is able to increase 28-day survival in patients with acute respiratory distress syndrome compared to conventional treatment (ARDSNet strategy).
To describe the data management process and statistical analysis plan.
The statistical analysis plan was designed by the trial executive committee and reviewed and approved by the trial steering committee. We provide an overview of the trial design with a special focus on describing the primary (28-day survival) and secondary outcomes. We describe our data management process, data monitoring committee, interim analyses, and sample size calculation. We describe our planned statistical analyses for primary and secondary outcomes as well as pre-specified subgroup analyses. We also provide details for presenting results, including mock tables for baseline characteristics, adherence to the protocol and effect on clinical outcomes.
According to best trial practice, we report our statistical analysis plan and data management plan prior to locking the database and beginning analyses. We anticipate that this document will prevent analysis bias and enhance the utility of the reported results.
ClinicalTrials.gov number, NCT01374022.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)