Abstract
Rev Bras Ter Intensiva. 2018;30(3):366-375
DOI 10.5935/0103-507X.20180049
To evaluate the effectiveness of rapid response teams using early identification of clinical deterioration in reducing the occurrence of in-hospital mortality and cardiorespiratory arrest.
The MEDLINE, LILACS, Cochrane Library, Center for Reviews and Dissemination databases were searched.
We included studies that evaluated the effectiveness of rapid response teams in adult hospital units, published in English, Portuguese, or Spanish, from 2000 to 2016; systematic reviews, clinical trials, cohort studies, and prepost ecological studies were eligible for inclusion. The quality of studies was independently assessed by two researchers using the Newcastle-Ottawa, modified Jadad, and Assessment of Multiple Systematic Reviews scales.
The results were synthesized and tabulated. When risk measures were reported by the authors of the included studies, we estimated effectiveness as 1-RR or 1-OR. In pre-post studies, we estimated effectiveness as the percent decrease in rates following the intervention.
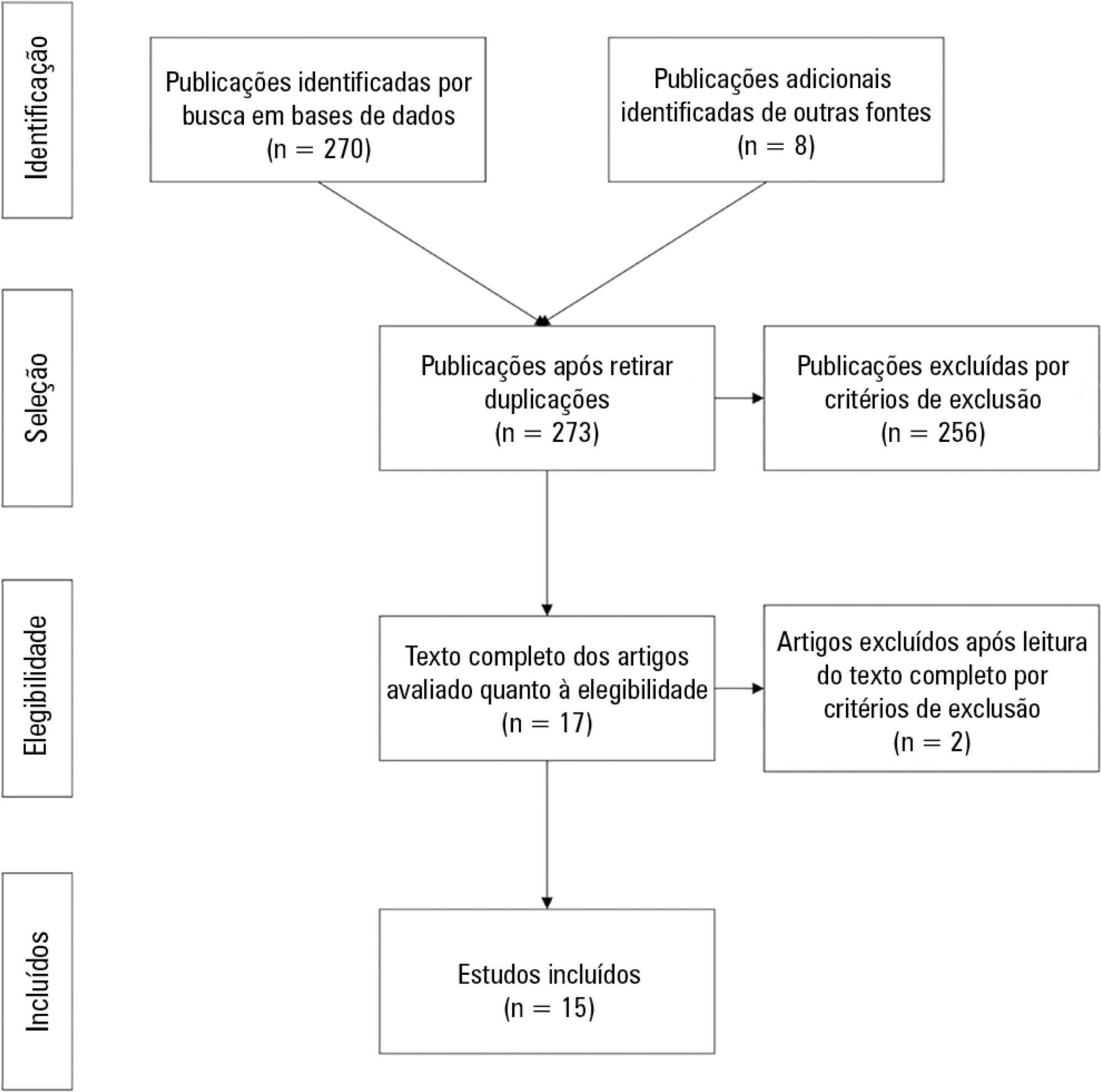
Overall, 278 studies were identified, 256 of which were excluded after abstract evaluation, and two of which were excluded after full text evaluation. In the meta-analysis of the studies reporting mortality data, we calculated a risk ratio of 0.85 (95%CI 0.76 - 0.94); and for studies reporting cardiac arrest data the estimated risk ratio was 0.65 (95%CI 0.49 - 0.87). Evidence was assessed as low quality due to the high heterogeneity and risk of bias in primary studies.
We conclude that rapid response teams may reduce in-hospital mortality and cardiac arrests, although the quality of evidence for both outcomes is low.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)