You searched for:"Marcelo Britto Passos Amato"
We found (2) results for your search.-
Original Article
A comprehensive physical functional assessment of survivors of critical care unit stay due to COVID-19
Crit Care Sci. 2024;36:e20240284en
Abstract
Original ArticleA comprehensive physical functional assessment of survivors of critical care unit stay due to COVID-19
Crit Care Sci. 2024;36:e20240284en
DOI 10.62675/2965-2774.20240284-en
Views12See moreABSTRACT
Objective:
To examine the physical function and respiratory muscle strength of patients – who recovered from critical COVID-19 – after intensive care unit discharge to the ward on Days one (D1) and seven (D7), and to investigate variables associated with functional impairment.
Methods:
This was a prospective cohort study of adult patients with COVID-19 who needed invasive mechanical ventilation, non-invasive ventilation or high-flow nasal cannula and were discharged from the intensive care unit to the ward. Participants were submitted to Medical Research Council sum-score, handgrip strength, maximal inspiratory pressure, maximal expiratory pressure, and short physical performance battery tests. Participants were grouped into two groups according to their need for invasive ventilation: the Invasive Mechanical Ventilation Group (IMV Group) and the Non-Invasive Mechanical Ventilation Group (Non-IMV Group).
Results:
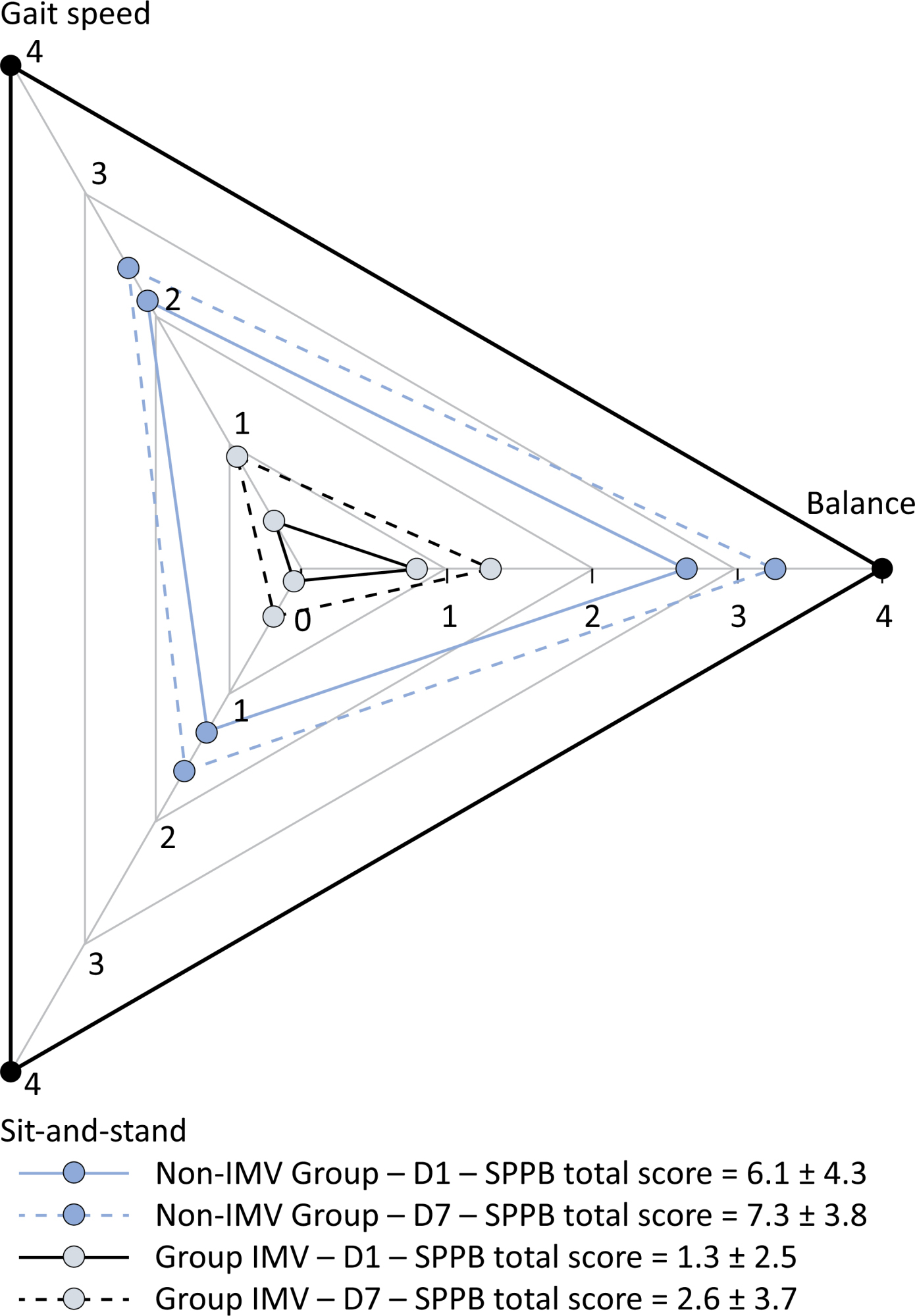
Patients in the IMV Group (n = 31) were younger and had higher Sequential Organ Failure Assessment scores than those in the Non-IMV Group (n = 33). The short physical performance battery scores (range 0 – 12) on D1 and D7 were 6.1 ± 4.3 and 7.3 ± 3.8, respectively for the Non-Invasive Mechanical Ventilation Group, and 1.3 ± 2.5 and 2.6 ± 3.7, respectively for the IMV Group. The prevalence of intensive care unit-acquired weakness on D7 was 13% for the Non-IMV Group and 72% for the IMV Group. The maximal inspiratory pressure, maximal expiratory pressure, and handgrip strength increased on D7 in both groups, but the maximal expiratory pressure and handgrip strength were still weak. Only maximal inspiratory pressure was recovered (i.e., > 80% of the predicted value) in the Non-IMV Group. Female sex, and the need and duration of invasive mechanical were independently and negatively associated with the short physical performance battery score and handgrip strength.
Conclusion:
Patients who recovered from critical COVID-19 and who received invasive mechanical ventilation presented greater disability than those who were not invasively ventilated. However, they both showed marginal functional improvement during early recovery, regardless of the need for invasive mechanical ventilation. This might highlight the severity of disability caused by SARS-CoV-2.
-
Original Articles
Statistical analysis plan for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART). A randomized controlled trial
Rev Bras Ter Intensiva. 2017;29(2):142-153
Abstract
Original ArticlesStatistical analysis plan for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART). A randomized controlled trial
Rev Bras Ter Intensiva. 2017;29(2):142-153
DOI 10.5935/0103-507X.20170024
Views0See moreABSTRACT
Background:
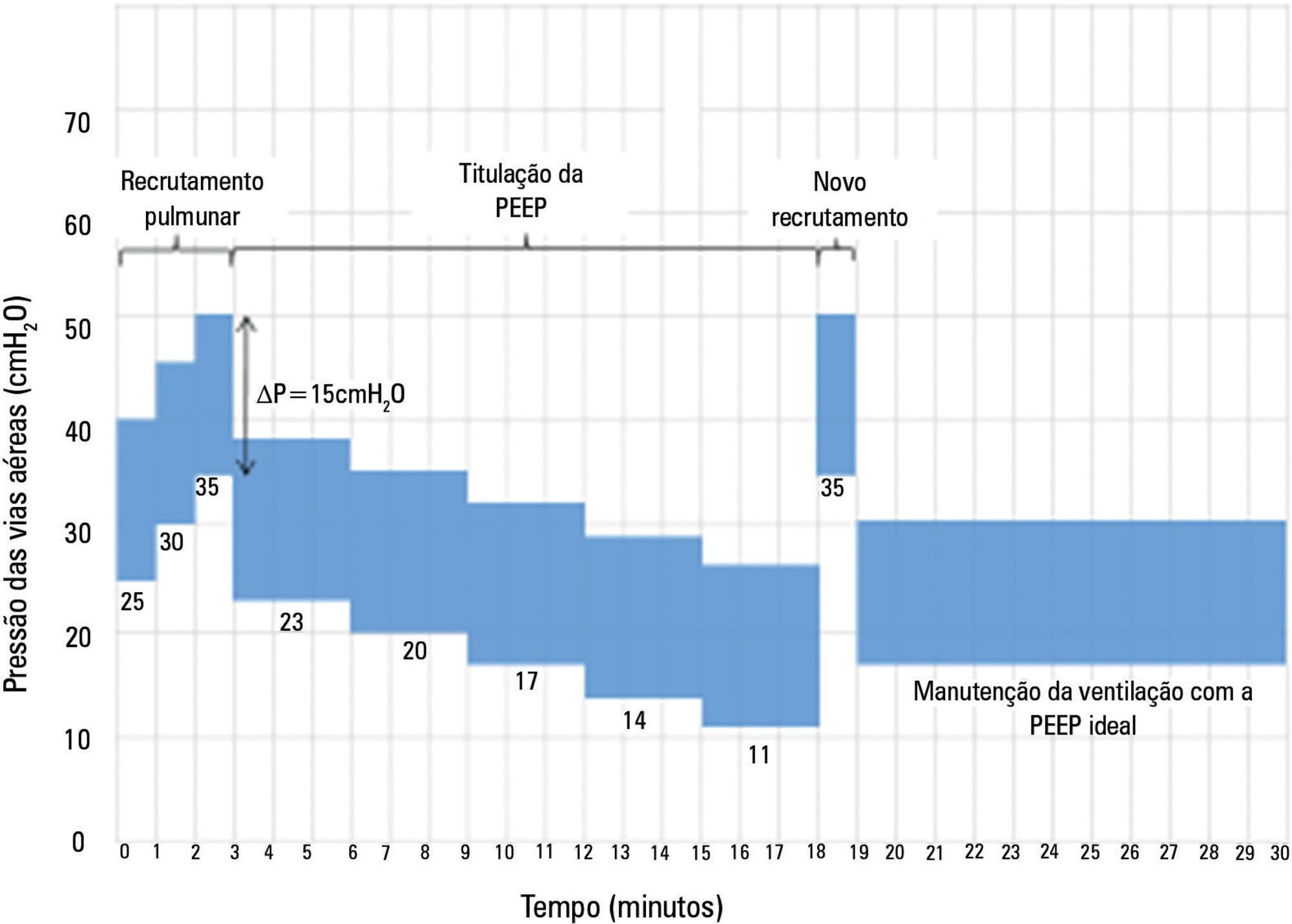
The Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) is an international multicenter randomized pragmatic controlled trial with allocation concealment involving 120 intensive care units in Brazil, Argentina, Colombia, Italy, Poland, Portugal, Malaysia, Spain, and Uruguay. The primary objective of ART is to determine whether maximum stepwise alveolar recruitment associated with PEEP titration, adjusted according to the static compliance of the respiratory system (ART strategy), is able to increase 28-day survival in patients with acute respiratory distress syndrome compared to conventional treatment (ARDSNet strategy).
Objective:
To describe the data management process and statistical analysis plan.
Methods:
The statistical analysis plan was designed by the trial executive committee and reviewed and approved by the trial steering committee. We provide an overview of the trial design with a special focus on describing the primary (28-day survival) and secondary outcomes. We describe our data management process, data monitoring committee, interim analyses, and sample size calculation. We describe our planned statistical analyses for primary and secondary outcomes as well as pre-specified subgroup analyses. We also provide details for presenting results, including mock tables for baseline characteristics, adherence to the protocol and effect on clinical outcomes.
Conclusion:
According to best trial practice, we report our statistical analysis plan and data management plan prior to locking the database and beginning analyses. We anticipate that this document will prevent analysis bias and enhance the utility of the reported results.
Trial registration:
ClinicalTrials.gov number, NCT01374022.
Search
Search in:
KEY WORDS
Case reports Child Coronavirus infections COVID-19 Critical care Critical illness ICU Infant, newborn Intensive care Intensive care units Intensive care units, pediatric mechanical ventilation Mortality Physical therapy modalities Prognosis Respiration, artificial Respiratory insufficiency risk factors SARS-CoV-2 Sepsis




