Abstract
Rev Bras Ter Intensiva. 2018;30(3):301-307
DOI 10.5935/0103-507X.20180043
To translate and cross-culturally adapt the Functional Status Scale for hospitalized children into Brazilian Portuguese.
A methodological study of the translation and cross-cultural adaptation of the Functional Status Scale was conducted, according to the stages of translation, synthesis of translations, back-translation, synthesis of back-translations, expert committee analysis and pre-test with a sample of the target population. During the evaluation by the committee of experts, semantic, content and item analyses were performed.
The semantic, idiomatic, cultural and conceptual equivalences between the translated version and the original version were obtained, resulting in the Brazilian version of the Functional Status Scale. After the analysis by the expert committee, there were no problems regarding the cultural or conceptual equivalences because the items were pertinent to the Brazilian culture and few terms were modified. In the pre-test stage, the scale was applied by two evaluators to a sample of 25 children. Clarity and ease in answering the scale items were observed. Good inter-observer reliability was obtained, with an intraclass correlation coefficient of 0.85 (0.59 - 0.95).
The Functional Status Scale for pediatric use was translated and culturally adapted into Portuguese spoken in Brazil. The translated items were pertinent to the Brazilian culture and evaluated the dimensions proposed by the original instrument. Validation studies of this instrument are suggested to make it feasible for use in different regions of Brazil.
Abstract
Rev Bras Ter Intensiva. 2018;30(2):181-186
DOI 10.5935/0103-507X.20180032
To evaluate the calibration and discrimination of APACHE IV in the postoperative period after kidney transplantation.
This clinical cohort study included 986 hospitalized adult patients in the immediate postoperative period after kidney transplantation, in a single center in southern Brazil.
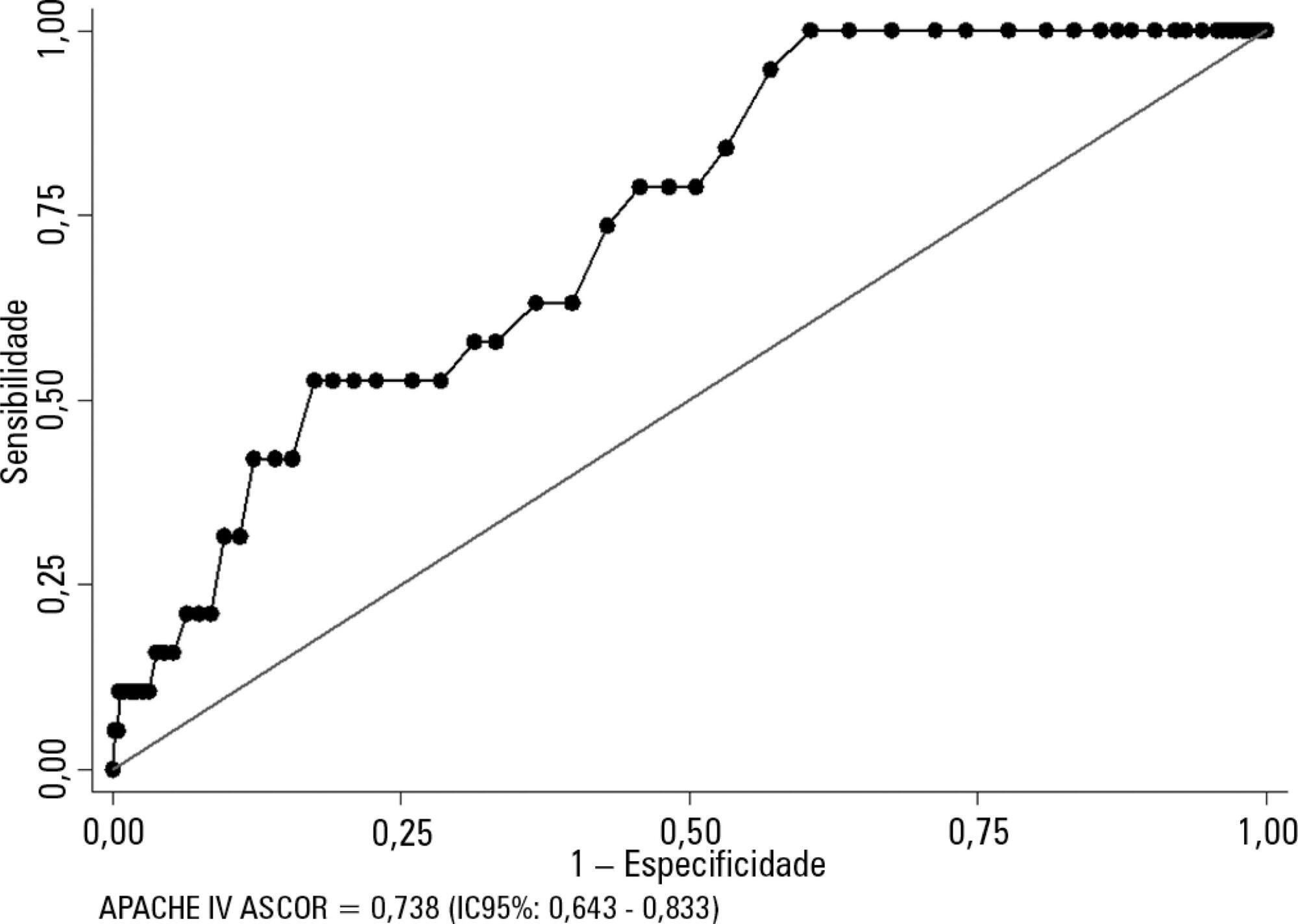
Kidney transplant patients who died in hospital had significantly higher APACHE IV values and higher predicted mortality. The APACHE IV score showed adequate calibration (H-L 11.24 p = 0.188) and a good discrimination ROC curve of 0.738 (95%CI 0.643 - 0.833, p < 0.001), although SMR overestimated mortality (SMR = 0.73; 95%CI: 0.24 - 1.42, p = 0.664).
The APACHE IV score showed adequate performance for predicting hospital outcomes in the postoperative period for kidney transplant recipients.
Abstract
Rev Bras Ter Intensiva. 2017;29(1):34-38
DOI 10.5935/0103-507X.20170006
The aim of the present study was to translate and cross-culturally adapt the Functional Status Score for the intensive care unit (FSS-ICU) into Brazilian Portuguese.
This study consisted of the following steps: translation (performed by two independent translators), synthesis of the initial translation, back-translation (by two independent translators who were unaware of the original FSS-ICU), and testing to evaluate the target audience's understanding. An Expert Committee supervised all steps and was responsible for the modifications made throughout the process and the final translated version.
The testing phase included two experienced physiotherapists who assessed a total of 30 critical care patients (mean FSS-ICU score = 25 ± 6). As the physiotherapists did not report any uncertainties or problems with interpretation affecting their performance, no additional adjustments were made to the Brazilian Portuguese version after the testing phase. Good interobserver reliability between the two assessors was obtained for each of the 5 FSS-ICU tasks and for the total FSS-ICU score (intraclass correlation coefficients ranged from 0.88 to 0.91).
The adapted version of the FSS-ICU in Brazilian Portuguese was easy to understand and apply in an intensive care unit environment.
Abstract
Rev Bras Ter Intensiva. 2014;26(3):287-291
DOI 10.5935/0103-507X.20140040
To develop experimental models of erythrocyte transfusion, the first step is to ensure the viability of the red blood cells transfused. In this pilot study, we assessed the viability of transfused red blood cells with validation in vitro and in vivo of homologous swine erythrocytes stored for 14 days.
Blood collected from one Agroceres® swine was stored in two red blood cell units. In vivo validation was performed by labeling the red blood cells with Na2 51CrO4 and recovering the viable erythrocytes after 24 hours of infusion in one autologous and four homologous animals. In vitro validation was performed at baseline and after 14 days in sixteen red blood cell units by measuring hemoglobin, hematocrit, hemolysis index and free hemoglobin. A post-mortem splenectomy was performed to evaluate the splenic sequestration of erythrocytes, and the radioactivity of the supernatant samples was counted to evaluate intravascular hemolysis.
After 14 days of storage, the red blood cell units had lower volumes and equivalent total concentrations of hemoglobin and hematocrit compared to human standards. The free hemoglobin concentration increased from 31.0±9.3 to 112.4±31.4mg/dL (p<0.001), and the hemolysis index increased from 0.1±0.1 to 0.5±0.1% (p<0.001). However, these tests were within the acceptable range for human standards. The percentage of radioactivity in supernatant samples was similar at baseline and after 24 hours, thus excluding significant hemolysis. No evidence of splenic sequestration of radioactive erythrocytes was found.
Swine red blood cells stored for 14 days are viable and can be used in experimental studies of transfusion. These validation experiments are important to aid investigators in establishing experimental models of transfusion.

Abstract
Rev Bras Ter Intensiva. 2013;25(2):106-114
DOI 10.5935/0103-507X.20130021
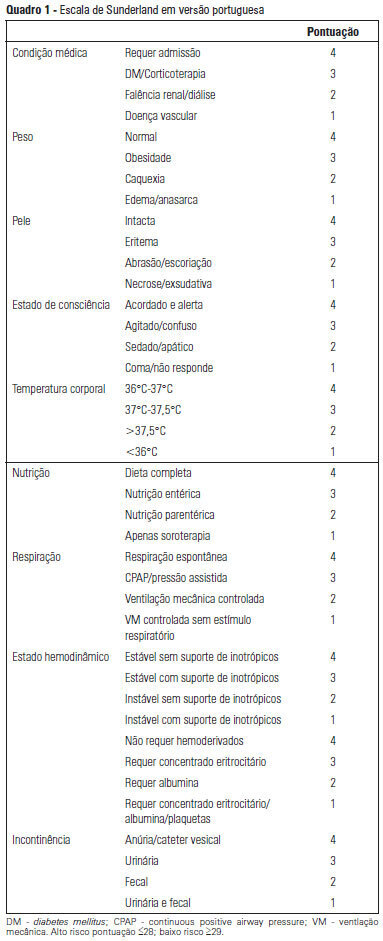
OBJECTIVE: To Translate into Portuguese and evaluate the measuring properties of the Sunderland Scale and the Cubbin & Jackson Revised Scale, which are instruments for evaluating the risk of developing pressure ulcers during intensive care. METHODS: This study included the process of translation and adaptation of the scales to the Portuguese language, as well as the validation of these tools. To assess the reliability, Cronbach alpha values of 0.702 to 0.708 were identified for the Sunderland Scale and the Cubbin & Jackson Revised Scale, respectively. The validation criteria (predictive) were performed comparatively with the Braden Scale (gold standard), and the main measurements evaluated were sensitivity, specificity, positive predictive value, negative predictive value, and area under the curve, which were calculated based on cutoff points. RESULTS: The Sunderland Scale exhibited 60% sensitivity, 86.7% specificity, 47.4% positive predictive value, 91.5% negative predictive value, and 0.86 for the area under the curve. The Cubbin & Jackson Revised Scale exhibited 73.3% sensitivity, 86.7% specificity, 52.4% positive predictive value, 94.2% negative predictive value, and 0.91 for the area under the curve. The Braden scale exhibited 100% sensitivity, 5.3% specificity, 17.4% positive predictive value, 100% negative predictive value, and 0.72 for the area under the curve. CONCLUSIONS: Both tools demonstrated reliability and validity for this sample. The Cubbin & Jackson Revised Scale yielded better predictive values for the development of pressure ulcers during intensive care.
Abstract
Rev Bras Ter Intensiva. 2010;22(2):175-185
DOI 10.1590/S0103-507X2010000200012
OBJECTIVE: The avoidance of pressure ulcers development in critically ill patients is a major nursing challenge. Prevention is thus relevant for assurance of high quality care. This study aimed to evaluate the applicability of the Braden scale in intensive care unit patients. METHODS: This was a prospective study based which evaluated all adult patients staying in the intensive care unit from July 14 to August 10, 2009. The data were collected using the Braden's scale by three examiners who identified the pressure ulcer development risk. The data were analyzed using the SAS Statistical Software. For determination of the examiners' rates degree of coincidence, the Kappa value was used (95%CI). RESULTS: Regarding the related risk factors: 36.4% had mild sensory perception impairment; 50.9% had occasionally moist skin; 97.3% bedfast; 39.1% had very limited mobility; 45% probably had inappropriate nutrition; 61.8% had friction and shear problems. An agreement between the examiners was identified for nutrition and physical activity (38.1% to 100.0%); the Kappa population zero hypothesis was rejected; a paired examiners agreement (41.7% to 100.0%) was identified for the items humidity and physical activity, and the Kappa values ranged from 0.13 to 1. CONCLUSIONS: These intensive care patients were identified to have increased risk of developing pressure ulcers. This tool was considered appropriate to support the implementation of preventive measures.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)