Abstract
Rev Bras Ter Intensiva. 2017;29(3):331-336
DOI 10.5935/0103-507X.20170040
The goal was to determine the main drug-related problems in neonates who were using antimicrobials.
This was an observational, prospective and longitudinal study. Drug-related problems were classified according to version 6.2 of the Pharmaceutical Care Network Europe Foundation classification. A descriptive analysis was performed, in which the clinical and therapeutic variables were presented as absolute and relative frequencies or as the mean and standard deviation, as appropriate.
In total, 152 neonates with a predominance of males (58.5%), gestational age of 32.7 ± 4.2 weeks and weight of 1,903.1 ± 846.9g were included. The main diagnostic hypothesis of infection was early sepsis (66.5%), and 71.7% of the neonates had some risk factor for infection. Among the neonates, 33.6% had at least one drug-related problem. Of these, 84.8% were related to treatment effectiveness and 15.2% to adverse reactions. The main cause of drug-related problems was the selected dose, particularly for aminoglycosides and cephalosporins.
The use of antimicrobials in the neonatal intensive care is mainly associated with problems related to medication effectiveness, predominantly the prescription of subdoses of antimicrobials, especially aminoglycosides.
Abstract
Rev Bras Ter Intensiva. 2012;24(2):151-156
DOI 10.1590/S0103-507X2012000200009
OBJECTIVE:To characterize drug prescriptions in a university hospital adult intensive care unit. METHODS: Single-center, observational, descriptive, cross-sectional study conducted at an adult general intensive care unit. The study population included all of the unit's inpatients from January to March 2011. The following characteristics for all prescriptions recorded during this period were examined: drug name (generic, brand name or abbreviation), dosage strength, pharmaceutical form, dose, route of administration, patient name, patient registration in the institution, clinic and hospital bed as well as the name, board license number, signature of the prescriber and date of the prescription. It was quantified the percentage of prescribed drugs included in the National List of Essential Drugs, the World Health Organization Model List of Essential Medicines and the University Hospital Center Pharmacotherapy Guide. The prescribed drugs were classified based on the Anatomical Therapeutic Chemical classification system (levels 1 and 2). RESULTS: Eight hundred forty-four prescriptions were reviewed from 72 patients (mean age: 59.04 ± 21.80), 54.92% of whom were female. The mean number of prescriptions per patient was 11.72 ± 11.68. The total number of drugs prescribed was 12,052 and 9,571 (79.41%) of the drugs were prescribed using the generic name. The most frequent absent information in the drug description was the pharmaceutical form of the drug (8,829/73.26%). The dosage strength was indicated in 7,231 (60%) of the prescriptions, and the prescriber and patient information were indicated in over 96% of the prescriptions. The prescribed drugs were classified in 13 therapeutic groups and 55 subgroups. Systemic antibacterials represented one of the most frequently prescribed subgroups. CONCLUSION: Most of the reviewed information was present in the prescriptions. However, the dosage strength and pharmaceutical form were absent in many prescriptions. The characterization of prescriptions at different hospital units is essential for the development of strategies that reduce drug utilization problems.
Abstract
Rev Bras Ter Intensiva. 2010;22(3):257-263
DOI 10.1590/S0103-507X2010000300007
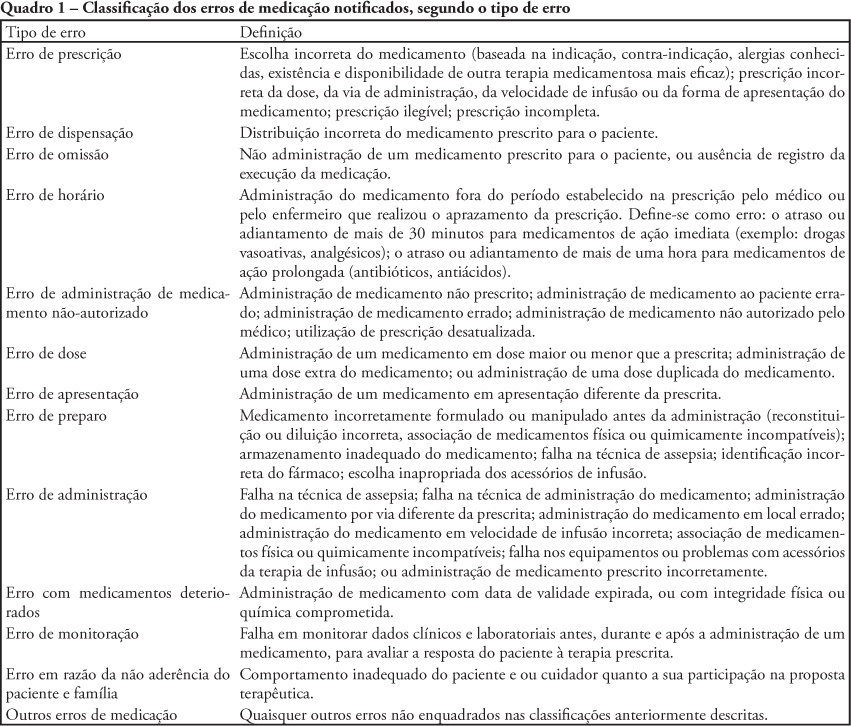
OBJECTIVE: To describe the frequency medication error disclosure to the team and to the family in an oncology pediatric patients' intensive care unit. METHODS: This was a descriptive and exploratory study performed between March 1 and May 31, 2008. A medication error report form was developed and implemented, to be completed by the professionals involved in the unit's medication process. RESULTS: The sample consisted of 71 forms collected over the 92 collection days. After medication error detection, the event was not reported to the pediatric intensive care unit's team in 34 (47.9%) cases. In the 37 reported to the team cases, for most of them (48.7%) the physician was the professional communicated. The event was not disclosed to the patient/family in 95.8% of medication error reports. CONCLUSIONS: Although the literature recommends disclosing the errors, this is not done in the studied pediatric intensive care unit.