Abstract
Crit Care Sci. 2023;35(1):57-65
DOI 10.5935/2965-2774.20230350-pt
To assess Brazilian pediatric intensivists’ general knowledge of extracorporeal membrane oxygenation, including evidence for its use, the national funding model, indications, and complications.
This was a multicenter cross-sectional survey including 45 Brazilian pediatric intensive care units. A convenience sample of 654 intensivists was surveyed regarding their knowledge on managing patients on extracorporeal membrane oxygenation, its indications, complications, funding, and literature evidence.
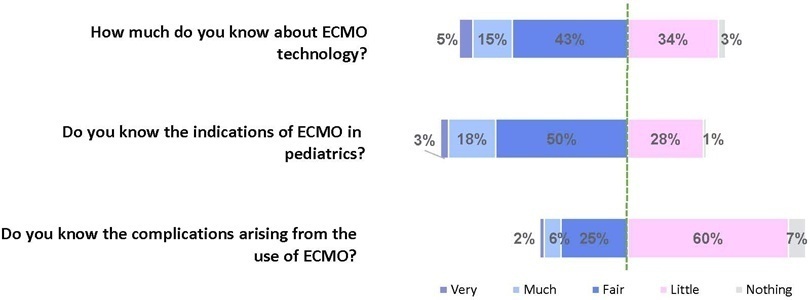
The survey addressed questions regarding the knowledge and experience of pediatric intensivists with extracorporeal membrane oxygenation, including two clinical cases and 6 optional questions about the management of patients on extracorporeal membrane oxygenation. Of the 45 invited centers, 42 (91%) participated in the study, and 412 of 654 (63%) pediatric intensivists responded to the survey. Most pediatric intensive care units were from the Southeast region of Brazil (59.5%), and private/for-profit hospitals represented 28.6% of the participating centers. The average age of respondents was 41.4 (standard deviation 9.1) years, and the majority (77%) were women. Only 12.4% of respondents had taken an extracorporeal membrane oxygenation course. Only 19% of surveyed hospitals have an extracorporeal membrane oxygenation program, and only 27% of intensivists reported having already managed patients on extracorporeal membrane oxygenation. Specific extracorporeal membrane oxygenation management questions were responded to by only 64 physicians (15.5%), who had a fair/good correct response rate (median 63.4%; range 32.8% to 91.9%).
Most Brazilian pediatric intensivists demonstrated limited knowledge regarding extracorporeal membrane oxygenation, including its indications and complications. Extracorporeal membrane oxygenation is not yet widely available in Brazil, with few intensivists prepared to manage patients on extracorporeal membrane oxygenation and even fewer intensivists recognizing when to refer patients to extracorporeal membrane oxygenation centers.
Abstract
Rev Bras Ter Intensiva. 2020;32(3):391-397
DOI 10.5935/0103-507X.20200067
To investigate the vancomycin effectiveness against gram-positive pathogens with the minimum inhibitory concentration of 1mg/L in pediatric patients based on the area under the curve and the minimum inhibitory concentration ratio > 400.
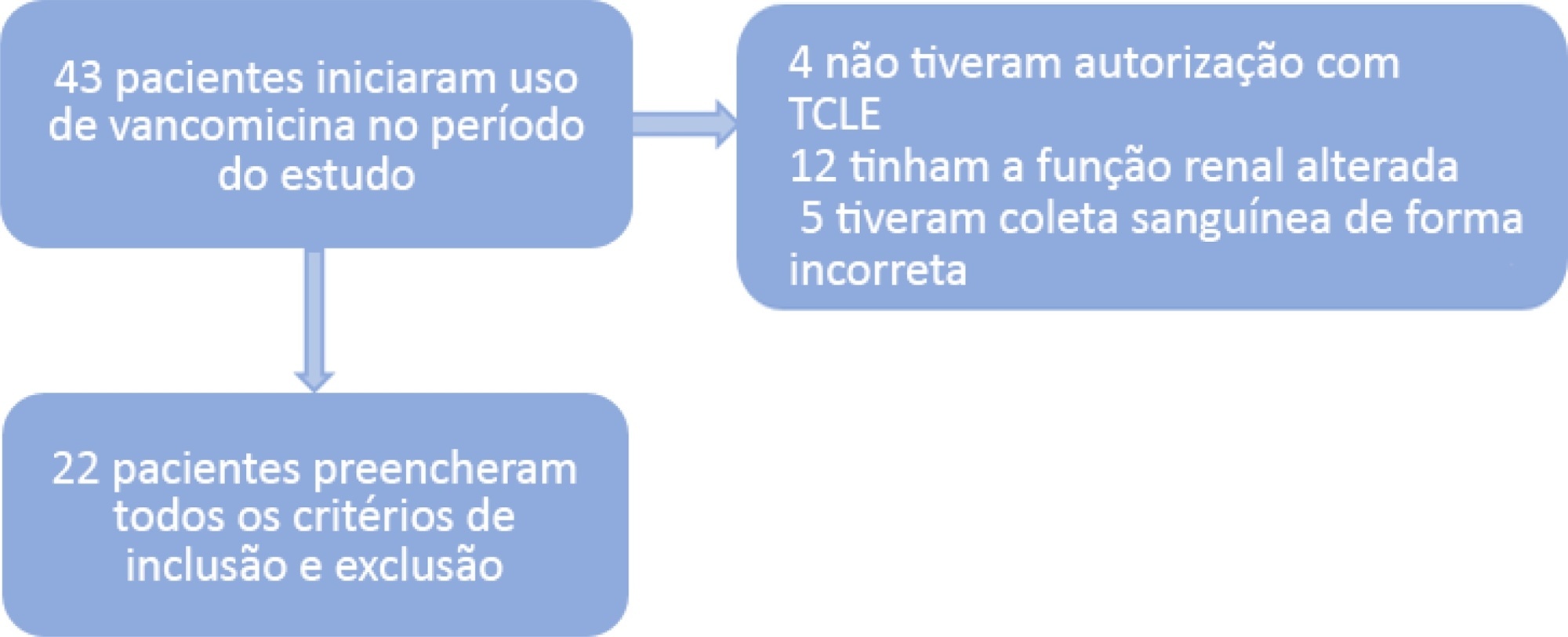
A population of 22 pediatric patients (13 boys) admitted to the pediatric intensive care unit with preserved renal function was stratified in two groups (G1 < 7 years and G2 ≥ 7 years). After the fourth dose administered of vancomycin (10 - 15mg/kg every 6 hours) was administered, two blood samples were collected (third and fifth hours), followed by serum measurement by immunoassays to investigate the pharmacokinetics and antimicrobial coverage.
There was no difference between the groups regarding dose, trough level or area under the curve. Coverage against gram-positive pathogens with a minimum inhibitory concentration of 1mg/L occurred in only 46% of patients in both groups. The pharmacokinetics in both groups were altered relative to the reference values, and the groups differed in regard to increased total body clearance and shortening of the biological half-life, which were more pronounced in younger patients.
A minimum empirical dose of 60mg/kg per day should be prescribed for pediatric patients in intensive care units with preserved renal function. The use of the ratio between the area under the curve and minimum inhibitory concentration in the evaluation of vancomycin coverage is recommended to achieve the desired outcome, since the pharmacokinetics are altered in these patients, which may impact the effectiveness of the antimicrobial.
Abstract
Rev Bras Ter Intensiva. 2010;22(3):257-263
DOI 10.1590/S0103-507X2010000300007
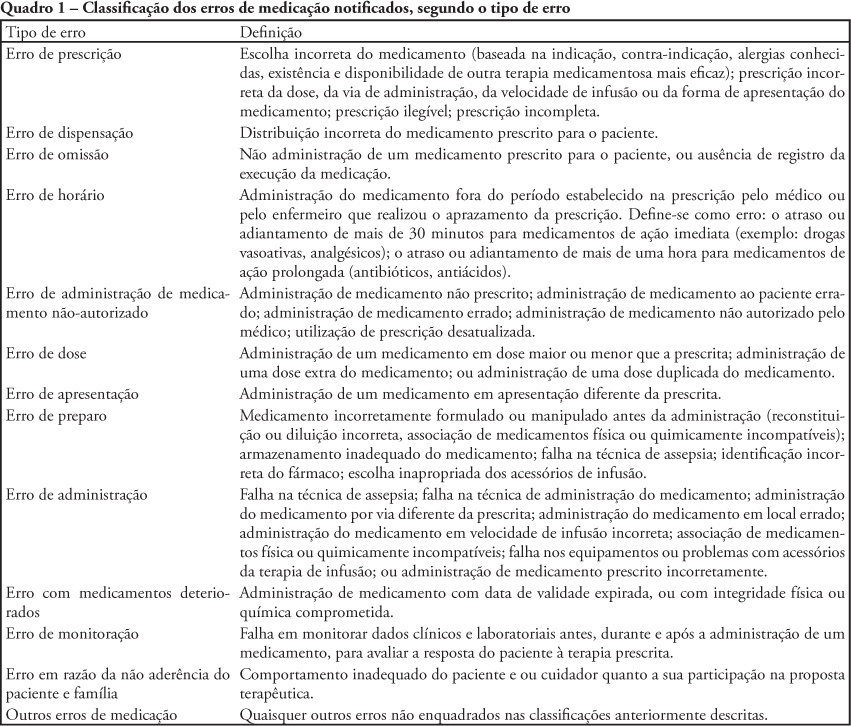
OBJECTIVE: To describe the frequency medication error disclosure to the team and to the family in an oncology pediatric patients' intensive care unit. METHODS: This was a descriptive and exploratory study performed between March 1 and May 31, 2008. A medication error report form was developed and implemented, to be completed by the professionals involved in the unit's medication process. RESULTS: The sample consisted of 71 forms collected over the 92 collection days. After medication error detection, the event was not reported to the pediatric intensive care unit's team in 34 (47.9%) cases. In the 37 reported to the team cases, for most of them (48.7%) the physician was the professional communicated. The event was not disclosed to the patient/family in 95.8% of medication error reports. CONCLUSIONS: Although the literature recommends disclosing the errors, this is not done in the studied pediatric intensive care unit.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)