You searched for:"Silvia Regina Cavani Jorge Santos"
We found (2) results for your search.-
Original Article
Vancomycin area under the curve-guided monitoring in pediatric patients
Rev Bras Ter Intensiva. 2022;34(1):147-153
Abstract
Original ArticleVancomycin area under the curve-guided monitoring in pediatric patients
Rev Bras Ter Intensiva. 2022;34(1):147-153
DOI 10.5935/0103-507X.20220009-en
Views1See moreABSTRACT
Objective:
To assess the percentage of vancomycin area under the curve/minimum inhibitory concentration target attainment in pediatric patients after the empirical dose regimen and to demonstrate the applicability of this method for vancomycin monitoring.
Methods:
A retrospective cohort study was performed including pediatric patients with normal renal function admitted between January 2020 and December 2020. The one-compartment model with first-order kinetics was used to estimate the pharmacokinetic parameters, and the area under the curve was calculated by the trapezoidal rule. The therapeutic target was defined as area under the curve/minimum inhibitory concentration ≥ 400 and < 600. The Chi-squared test was applied to compare the percentage of target attainment over age groups, while the pharmacokinetic parameters were compared by the Kruskal-Wallis test with Dunn’s test for post hoc analyses. We considered significant p-values < 0.05.
Results:
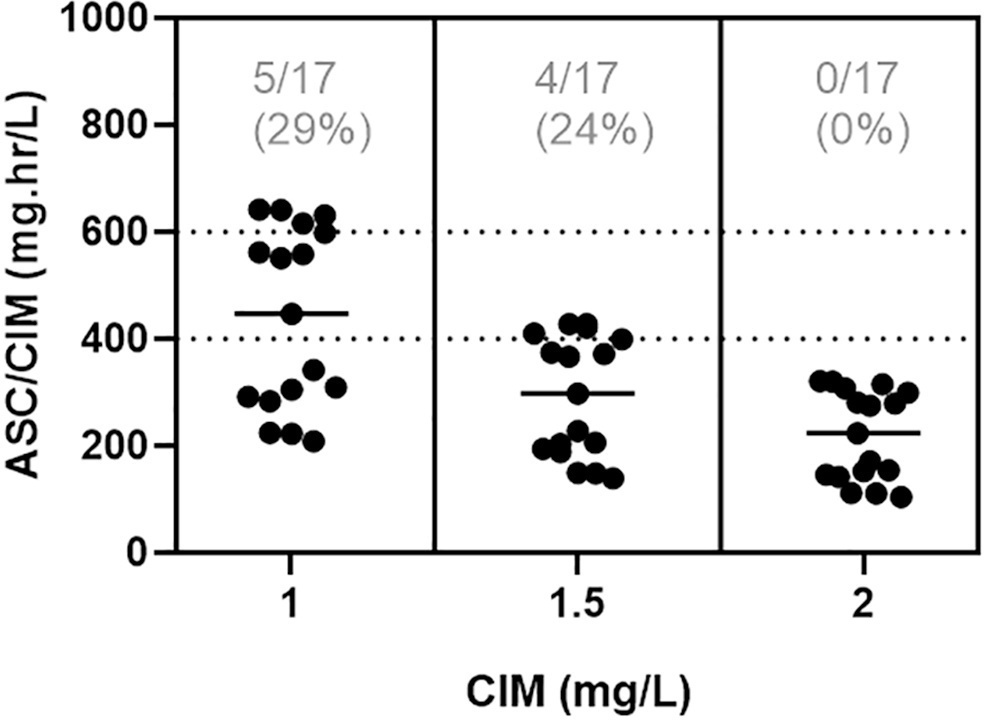
In total, 42 pairs of vancomycin levels were analyzed from 17 patients enrolled in this study. After empirical vancomycin daily dosing, the therapeutic target was achieved in five (29%) patients; four patients (24%) had a supratherapeutic initial area under the curve/minimum inhibitory concentration value (> 600mg.h/L), and eight (47%) patients had subtherapeutic values (< 400mg.h/L). The most identified pathogens were Staphylococcus spp. (n = 7). Trough levels and areas under the curve showed moderate correlation values (R2 = 0.73). Acute kidney injury occurred in one (6%) patient.
Conclusion:
Most patients did not reach the therapeutic target with a vancomycin empirical dose regimen, and the implementation of area under the curve-based dosing using two sample measurements allowed for real-time dose adjustments based on individuals’ pharmacokinetic parameters.
-
Original Article
Does vancomycin administered at an empirical dose ensure coverage of pediatric patients against gram-positive pathogens?
Rev Bras Ter Intensiva. 2020;32(3):391-397
Abstract
Original ArticleDoes vancomycin administered at an empirical dose ensure coverage of pediatric patients against gram-positive pathogens?
Rev Bras Ter Intensiva. 2020;32(3):391-397
DOI 10.5935/0103-507X.20200067
Views0Abstract
Objective:
To investigate the vancomycin effectiveness against gram-positive pathogens with the minimum inhibitory concentration of 1mg/L in pediatric patients based on the area under the curve and the minimum inhibitory concentration ratio > 400.
Methods:
A population of 22 pediatric patients (13 boys) admitted to the pediatric intensive care unit with preserved renal function was stratified in two groups (G1 < 7 years and G2 ≥ 7 years). After the fourth dose administered of vancomycin (10 - 15mg/kg every 6 hours) was administered, two blood samples were collected (third and fifth hours), followed by serum measurement by immunoassays to investigate the pharmacokinetics and antimicrobial coverage.
Results:
There was no difference between the groups regarding dose, trough level or area under the curve. Coverage against gram-positive pathogens with a minimum inhibitory concentration of 1mg/L occurred in only 46% of patients in both groups. The pharmacokinetics in both groups were altered relative to the reference values, and the groups differed in regard to increased total body clearance and shortening of the biological half-life, which were more pronounced in younger patients.
Conclusion:
A minimum empirical dose of 60mg/kg per day should be prescribed for pediatric patients in intensive care units with preserved renal function. The use of the ratio between the area under the curve and minimum inhibitory concentration in the evaluation of vancomycin coverage is recommended to achieve the desired outcome, since the pharmacokinetics are altered in these patients, which may impact the effectiveness of the antimicrobial.
Keywords:ChildDrug monitoringPediatric intensive care unitsPharmacokineticsPharmacologic actionsVancomycin/administration & dosageSee more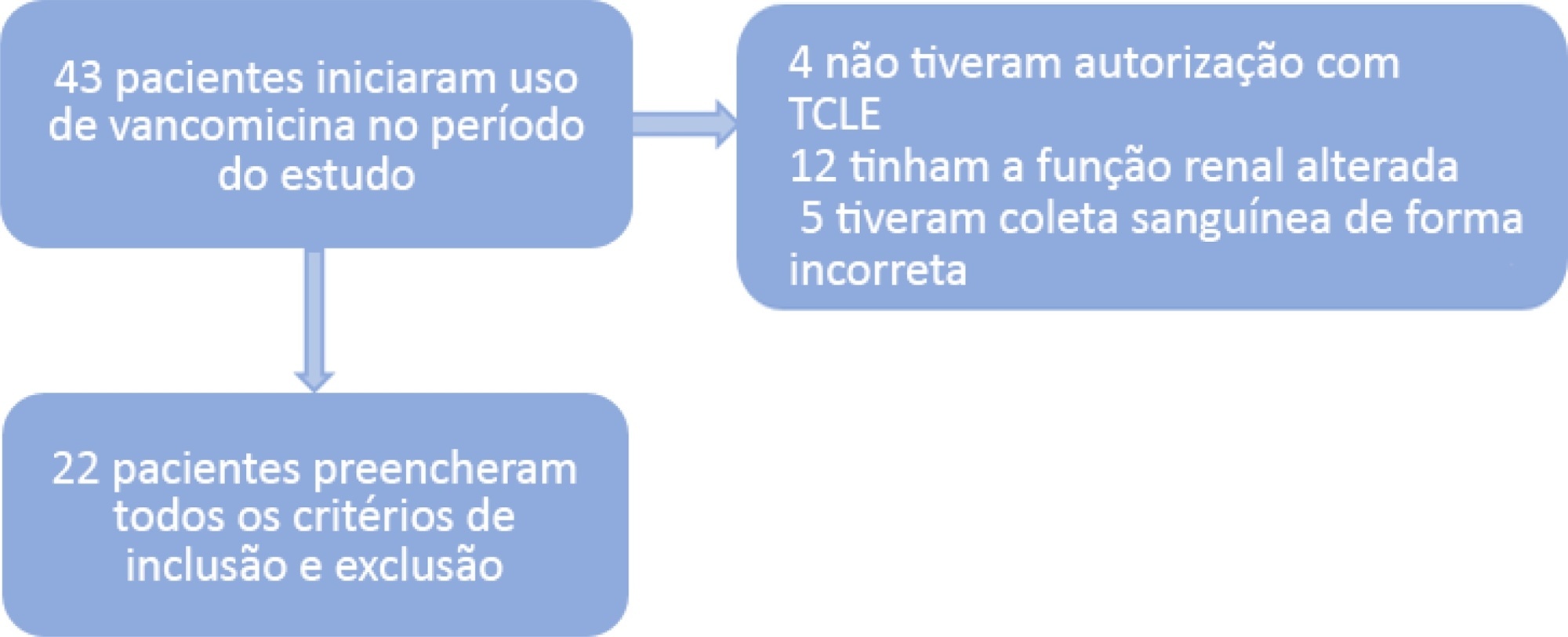
Search
Search in:
KEY WORDS
Case reports Child Coronavirus infections COVID-19 Critical care Critical illness Extracorporeal membrane oxygenation Infant, newborn Intensive care Intensive care units Intensive care units, pediatric mechanical ventilation Mortality Physical therapy modalities Prognosis Respiration, artificial Respiratory insufficiency risk factors SARS-CoV-2 Sepsis




