Abstract
Rev Bras Ter Intensiva. 2021;33(4):600-615
DOI 10.5935/0103-507X.20210087
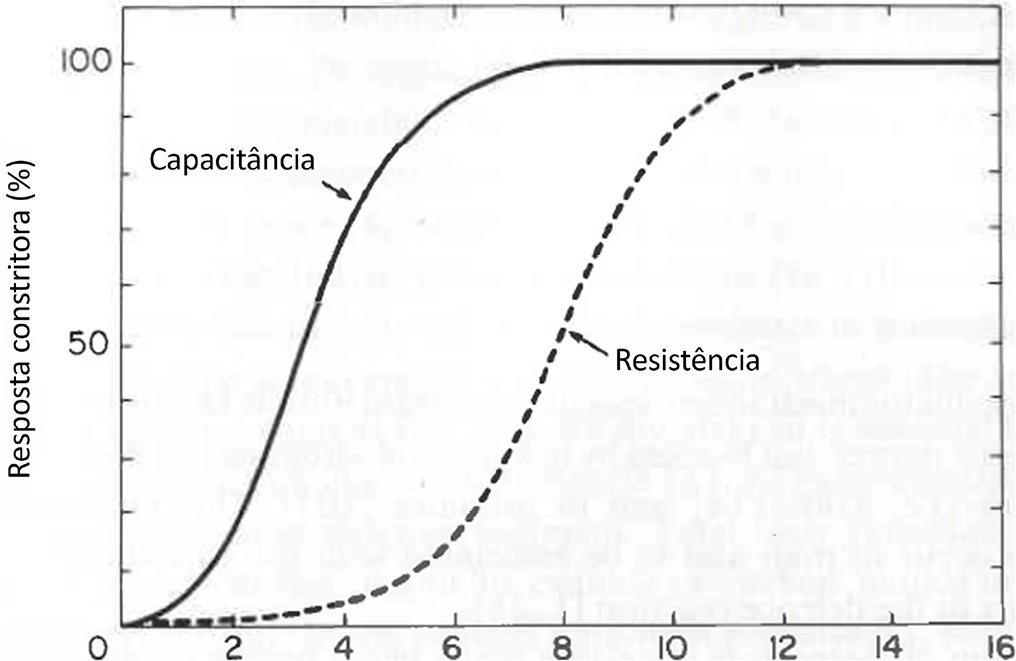
Cardiac, ventilatory and kidney management in the critical care setting has been optimized over the past decades. Cognition and sedation represent one of the last remaning challenges. As conventional sedation is suboptimal and as the sedation evoked by alpha-2 adrenergic agonists (“cooperative” sedation with dexmedetomidine, clonidine or guanfacine) represents a valuable alternative, this manuscript covers three practical topics for which evidence-based medicine is lacking: a) Switching from conventional to cooperative sedation (“switching”): the short answer is the abrupt withdrawal of conventional sedation, immediate implementation of alpha-2 agonist infusion and the use of “rescue sedation” (midazolam bolus[es]) or “breakthrough sedation” (haloperidol bolus[es]) to stabilize cooperative sedation. b) Switching from conventional to cooperative sedation in unstable patients (e.g., refractory delirium tremens, septic shock, acute respiratory distress syndrome, etc.): to avoid hypotension and bradycardia evoked by sympathetic deactivation, the short answer is to maintain the stroke volume through volume loading, vasopressors and inotropes. c) To avoid these switches and associated difficulties, alpha-2 agonists may be considered first-line sedatives. The short answer is to administer alpha-2 agonists slowly from admission or endotracheal intubation up to stabilized cooperative sedation. The “take home” message is as follows: a) alpha-2 agonists are jointly sympathetic deactivators and sedative agents; b) sympathetic deactivation implies maintaining the stroke volume and iterative assessment of volemia. Evidence-based medicine should document our propositions.
Abstract
Rev Bras Ter Intensiva. 2008;20(1):24-30
DOI 10.1590/S0103-507X2008000100004
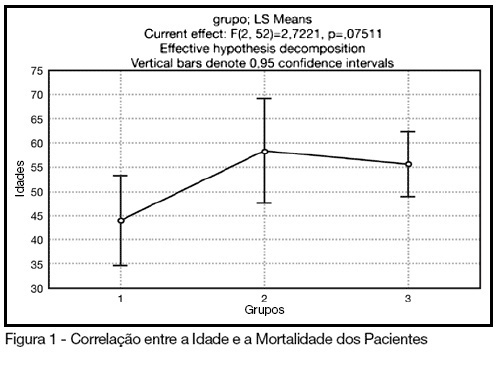
BACKGROUND AND OBJECTIVES: The control of patient discomfort in the intensive care unit (ICU) has become an integral part of critical care practice. The sedoanalgesic drugs could influence critically ill patient's morbimortality. Alpha²-adrenoceptor agonists might have an interesting future in ICU. The objective of this study is to evaluate the clonidine use for sedoanalgesia in ICU patients under prolonged mechanical ventilation. METHODS: Historical cohort study. Admitted patient files, January-December 2006, which stayed under mechanical ventilation, analgesia and sedation > 7days were analyzed. Demographic, clinical features and therapeutic data concerning analgesia and sedation were remarked. The data allowed classify the patients in three different groups: G1 = patients that used clonidine and other drugs; G2 = patients that used three or more drugs, without clonidine and G3 = patients that used fentanyl and midazolam. The mean daily doses of infused clonidine were registered, and the values of heat rate (HR), blood arterial pressure (BAP) before starting use of clonidine, after six hours and 24 hours were also registered. Statistical analyzes were performed using Variance Analysis (ANOVA), t tests and x² (significance p < 0.05). RESULTS: Were selected 55 patients. Fifteen (27.2%) belonged to G1, 11 (20%) belonged to G2 and 29 (52.7%) belonged to G3. The mean age of patients was 44 (G1), 50 (G2) and 56 (G3) (p = NS). The mean score APACHE II was 18 (G1), 20.4 (G2) and 20.7 (G3) (p = NS). G1 and G2 patients presented higher ICU length-of-stay (p < 0.05). The mean dose of clonidine used was 1.21 ± 0.54 mg/kg/min. G1 patients had HR and BAP decreased, however these effects were not considered clinically relevant. The mortality was lower in the patients from G1 (20%) when compared to G2 (54.5%) and G3 (62%) (p < 0.05). CONCLUSIONS: The clonidine use to analyzed patients did not result in clinical relevant side effects. The lower mortality index in patients that used clonidine was statistical significant.
Abstract
Rev Bras Ter Intensiva. 2007;19(3):284-291
DOI 10.1590/S0103-507X2007000300003
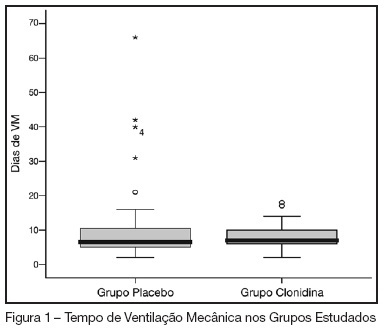
BACKGROUND AND OBJECTIVES: In our country, the abstinence syndrome has high prevalence and besides the distress prolongs the length of hospital stay. The aim of this study was to evaluate the influence of associating oral clonidine to the intravenous morphine plus midazolam continuous infusion in children submitted to mechanical ventilation. METHODS: Randomized, double blind, placebo controlled study, conducted in the PICU at the Hospital Geral of Caxias do Sul. All children (1-36 months) submitted to mechanical ventilation over 12 hours (May-2005/August-2006), which had used intravenous morphine and midazolam continuous infusion were included. They were randomized to received clonidine (5 µg/kg) or placebo associated to the sedative continuous infusion. The infusion sedative doses were at the discretion of the assistant physician. The administered doses in the previous 24 hours and the doses of intermittent sedation were daily collected. The abstinence syndrome was defined based on Finnegan Score. The groups were compared regarding the cumulative doses of sedatives, length of use of sedative continuous infusion, presence and duration of the abstinence. RESULTS: Were included 69 patients (31 in the clonidine group and 38 in the placebo group). The two groups were similar regarding the general characteristics (weight, age, gender, indication of mechanical ventilation). The midazolam and morphine doses (cumulative and intermittent doses) were not different in both groups. Completed the study 59 patients, 25 in clonidine group and 34 in placebo group. The prevalence of the abstinence was similar (72% and 75%, respectively), without difference in the recovery time neither related to the length of mechanical ventilation. CONCLUSIONS: In children submitted to mechanical ventilation using high dose of opioids and diazepinic infusion the addiction of clonidine did not decrease the daily doses neither the cumulative doses and nevertheless reduced the prevalence and the evolution of abstinence syndrome.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)