Abstract
Rev Bras Ter Intensiva. 2017;29(3):310-316
DOI 10.5935/0103-507X.20170039
To phenotypically evaluate biofilm production by Pseudomonas aeruginosa clinically isolated from patients with ventilator-associated pneumonia.
Twenty clinical isolates of P. aeruginosa were analyzed, 19 of which were from clinical samples of tracheal aspirate, and one was from a bronchoalveolar lavage sample. The evaluation of the capacity of P. aeruginosa to produce biofilm was verified using two techniques, one qualitative and the other quantitative.
The qualitative technique showed that only 15% of the isolates were considered biofilm producers, while the quantitative technique showed that 75% of the isolates were biofilm producers. The biofilm isolates presented the following susceptibility profile: 53.3% were multidrug-resistant, and 46.7% were multidrug-sensitive.
The quantitative technique was more effective than the qualitative technique for the detection of biofilm production. For the bacterial population analyzed, biofilm production was independent of the susceptibility profile of the bacteria, demonstrating that the therapeutic failure could be related to biofilm production, as it prevented the destruction of the bacteria present in this structure, causing complications of pneumonia associated with mechanical ventilation, including extrapulmonary infections, and making it difficult to treat the infection.
Abstract
Rev Bras Ter Intensiva. 2015;27(3):260-265
DOI 10.5935/0103-507X.20150047
>To evaluate the agreement between a new epidemiological surveillance method of the Center for Disease Control and Prevention and the clinical pulmonary infection score for mechanical ventilator-associated pneumonia detection.
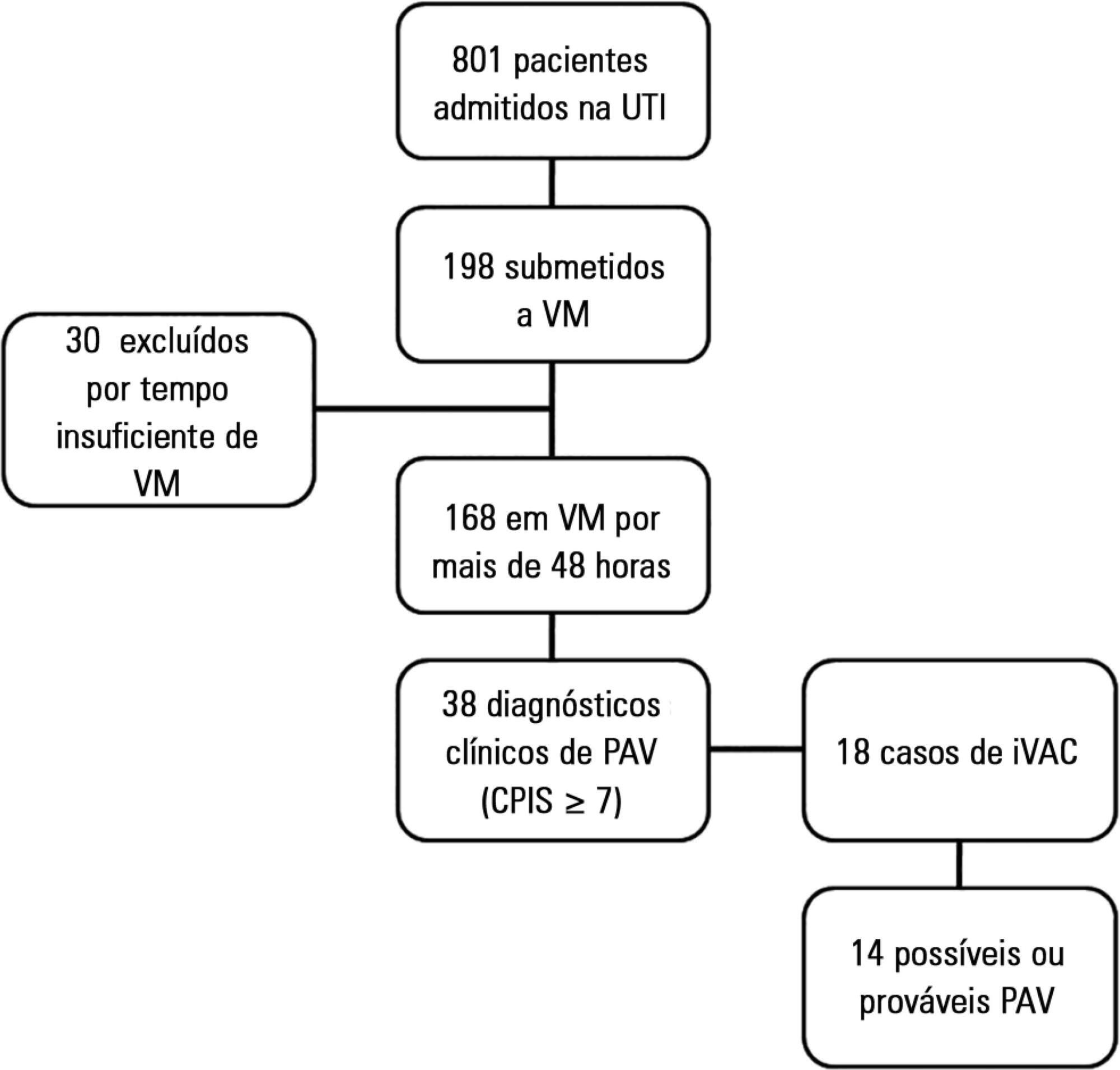
This was a prospective cohort study that evaluated patients in the intensive care units of two hospitals who were intubated for more than 48 hours between August 2013 and June 2014. Patients were evaluated daily by physical therapist using the clinical pulmonary infection score. A nurse independently applied the new surveillance method proposed by the Center for Disease Control and Prevention. The diagnostic agreement between the methods was evaluated. A clinical pulmonary infection score of ≥ 7 indicated a clinical diagnosis of mechanical ventilator-associated pneumonia, and the association of a clinical pulmonary infection score ≥ 7 with an isolated semiquantitative culture consisting of ≥ 104 colony-forming units indicated a definitive diagnosis.
Of the 801 patients admitted to the intensive care units, 198 required mechanical ventilation. Of these, 168 were intubated for more than 48 hours. A total of 18 (10.7%) cases of mechanical ventilation-associated infectious conditions were identified, 14 (8.3%) of which exhibited possible or probable mechanical ventilatorassociated pneumonia, which represented 35% (14/38) of mechanical ventilator-associated pneumonia cases. The Center for Disease Control and Prevention method identified cases of mechanical ventilator-associated pneumonia with a sensitivity of 0.37, specificity of 1.0, positive predictive value of 1.0, and negative predictive value of 0.84. The differences resulted in discrepancies in the mechanical ventilator-associated pneumonia incidence density (CDC, 5.2/1000 days of mechanical ventilation; clinical pulmonary infection score ≥ 7, 13.1/1000 days of mechanical ventilation).
The Center for Disease Control and Prevention method failed to detect mechanical ventilatorassociated pneumonia cases and may not be satisfactory as a surveillance method.