Abstract
Rev Bras Ter Intensiva. 2019;31(2):193-201
DOI 10.5935/0103-507X.20190033
To characterize resource availability from a nationally representative random sample of intensive care units in Brazil.
A structured online survey of participating units in the Sepsis PREvalence Assessment Database (SPREAD) study, a nationwide 1-day point prevalence survey to assess the burden of sepsis in Brazil, was sent to the medical director of each unit.
A representative sample of 277 of the 317 invited units responded to the resources survey. Most of the hospitals had fewer than 500 beds (94.6%) with a median of 14 beds in the intensive care unit. Providing care for public-insured patients was the main source of income in two-thirds of the surveyed units. Own microbiology laboratory was not available for 26.8% of the surveyed intensive care units, and 10.5% did not always have access to blood cultures. Broad spectrum antibiotics were not always available in 10.5% of surveyed units, and 21.3% could not always measure lactate within three hours. Those institutions with a high resource availability (158 units, 57%) were usually larger and preferentially served patients from the private health system compared to institutions without high resource availability. Otherwise, those without high resource availability did not always have broad-spectrum antibiotics (24.4%), vasopressors (4.2%) or crystalloids (7.6%).
Our study indicates that a relevant number of units cannot perform basic monitoring and therapeutic interventions in septic patients. Our results highlight major opportunities for improvement to adhere to simple but effective interventions in Brazil.
Abstract
Rev Bras Ter Intensiva. 2011;23(1):87-95
DOI 10.1590/S0103-507X2011000100014
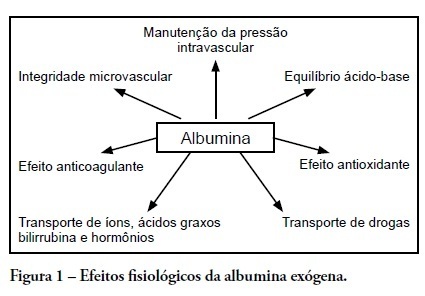
Human albumin has been used as a therapeutic agent in intensive care units for more than 50 years. However, clinical studies from the late 1990s described possible harmful effects in critically ill patients. These studies' controversial results followed other randomized controlled studies and meta-analyses that showed no harmful effects of this colloid solution. In Brazil, several public and private hospitals comply with the Agência Nacional de Vigilância Sanitária (the Brazilian Health Surveillance Agency) recommendations for appropriate administration of intravenous albumin. This review discusses indications for albumin administration in critically ill patients and analyzes the evidence for metabolic and immunomodulatory effects of this colloid solution. We also describe the most significant studies from 1998 to the present time; these reveal an absence of incremental mortality from intravenous albumin administration as compared to crystalloid solutions. The National Health Surveillance Agency indications are discussed relative to the current body of evidence for albumin use in critically ill patients.
Search
Search in:
Case reports (56) Child (53) Coronavirus infections (34) COVID-19 (46) Critical care (116) Critical illness (54) Extracorporeal membrane oxygenation (26) Infant, newborn (27) Intensive care (72) Intensive care units (256) Intensive care units, pediatric (31) mechanical ventilation (38) Mortality (76) Physical therapy modalities (28) Prognosis (61) Respiration, artificial (119) Respiratory insufficiency (26) risk factors (34) SARS-CoV-2 (28) Sepsis (98)