Abstract
Rev Bras Ter Intensiva. 2012;24(4):369-374
DOI 10.1590/S0103-507X2012000400013
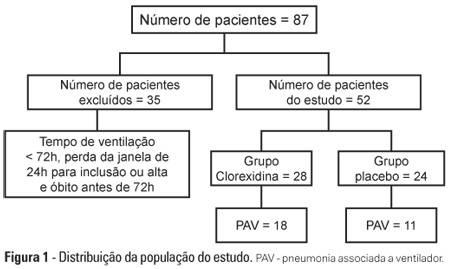
OBJECTIVE: To evaluate the effects of oral chlorhexidine hygiene with toothbrushing on the rate of ventilator-associated pneumonia in a mixed population of critically ill patients under prolonged mechanical ventilation. METHODS: Prospective, randomized, and placebo-controlled pilot study. Patients who were receiving mechanical ventilation, had been admitted less than 24 hours prior, and were anticipated to require mechanical ventilation for more than 72 hours were included in the study. The patients were randomly divided into one of the following groups: chlorhexidine hygiene with toothbrushing or a placebo group (gel with the same color and consistency and toothbrushing). RESULTS: The planned interim analysis was conducted using 52 patients, and the study was terminated prematurely. In total, 28 patients were included in the chlorhexidine / toothbrushing group, and 24 patients were included in the placebo group. Ventilator-associated pneumonia occurred in 45.8% of the placebo group and in 64.3% of the chlorhexidine hygiene with toothbrushing group (RR=1.4; 95% CI=0.83-2.34; p=0.29). CONCLUSION: Because the study was terminated due to futility, it was not possible to evaluate the impact of oral hygiene using 2% chlorhexidine and toothbrushing on the incidence of ventilator-associated pneumonia in this heterogeneous population of critical patients receiving long-term mechanical ventilation, and no beneficial effect was observed for this intervention.