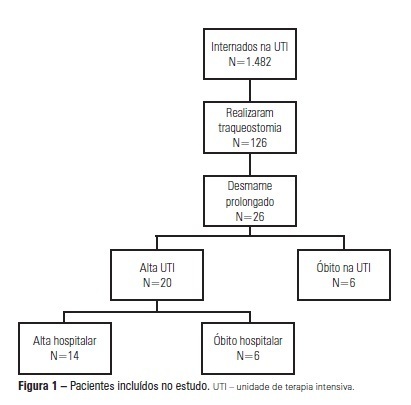
OBJECTIVE: We aimed to assess the use of noninvasive ventilation devices in patients with prolonged weaning following tracheotomy. METHODS: We performed a retrospective observational study using data collected from the clinical records of tracheotomized patients diagnosed with prolonged weaning. The participants were hospitalized in the adult intensive care unit of Moinhos de Vento Hospital, Porto Alegre (RS) between December 2007 and December 2008. RESULTS: In the data collection period, 1,482 patients were admitted to the intensive care unit. In total, 126 patients underwent tracheotomies, and 26 of these patients met the inclusion criteria for participating in the study. The average age of the patients in our sample was 73 ± 12 years. In our sample, 57.7% of the participants were female, and 80.8% were admitted as a result of acute hypoxemic respiratory failure. After the tracheotomy, the patients remained under mechanical ventilation for an average of 29.8 days. After the initiation of the experimental protocol, the tracheotomized patients remained under ventilation for an average of 53.5 days on a portable noninvasive device connected to the tracheotomy. There were three possible outcomes for the patients. They were discharged, were weaned from the noninvasive ventilation, or died in the intensive care unit or hospital ward. In total, 76.9% (20/26) of the patients were discharged from the intensive care unit, and 53.8% (14/26) of the patients were discharged from the hospital. CONCLUSION: The use of noninvasive portable ventilators connected to the tracheotomy may represent an alternative for discontinuing ventilationand discharging tracheotomized patients with prolonged ventilatory weaning from intensive care unit.
Search
Search in:


Comments