To evaluate the occurrence of adverse events in the postoperative period of cardiac surgery in a pediatric intensive care unit and to find any patient characteristics that can predict such events.
This was a historical cohort study of patients recovering in the pediatric intensive care unit for the first 7 days after cardiac surgery between April and December 2019, by reviewing the medical records. The following were reviewed: demographic, clinical, and laboratory characteristics; patient severity scores; and selected adverse events, grouped into device-related, surgical, and nonsurgical.
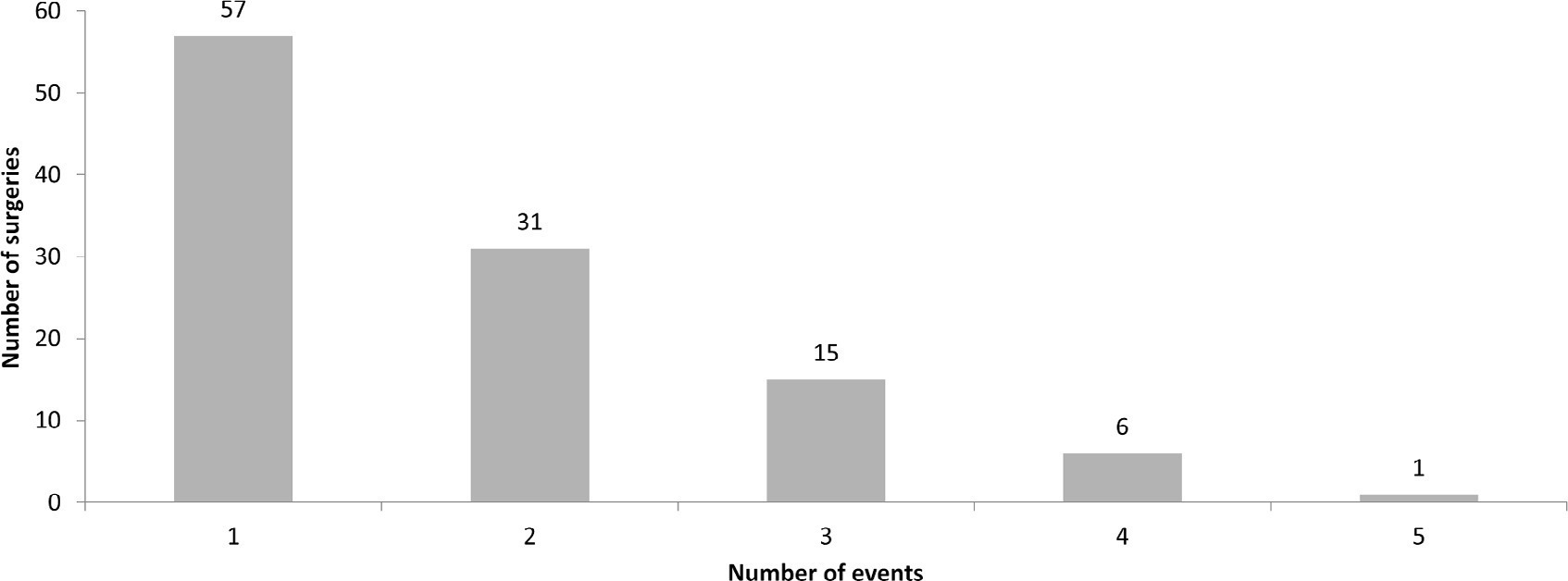
A total of 238 medical records were included. At least one adverse event occurred in 110 postoperative patients (46.2%). The total number of adverse events was 193 (81%). Vascular catheters were the most common cause, followed by cardiac arrest, bleeding, and surgical reexploration. In the univariate analysis, the vasoactive-inotropic score (VIS), Risk Adjustment in Congenital Heart Surgery (RACHS-1) score, age, Pediatric Index of Mortality (PIM-2), cardiopulmonary bypass and aortic clamping duration were significantly associated with adverse events. In the multivariate analysis, VIS ≥ 20 (OR 2.90; p = 0.004) and RACHS-1 ≥ 3 (OR 2.11; p = 0.019) were significant predictors, while age and delayed sternal closure showed only trends toward significance. To predict the occurrence of adverse events from VIS and RACHS-1, the area under the curve was 0.73 (95%CI 0.66 – 0.79).
Adverse events were quite frequent in children after cardiac surgery, especially those related to devices. The VIS and RACHS-1, used together, predicted the occurrence of adverse events well in this pediatric sample.
Search
Search in:


Comments