To determine micafungin plasma levels and pharmacokinetic behavior in patients treated with extracorporeal membrane oxygenation.
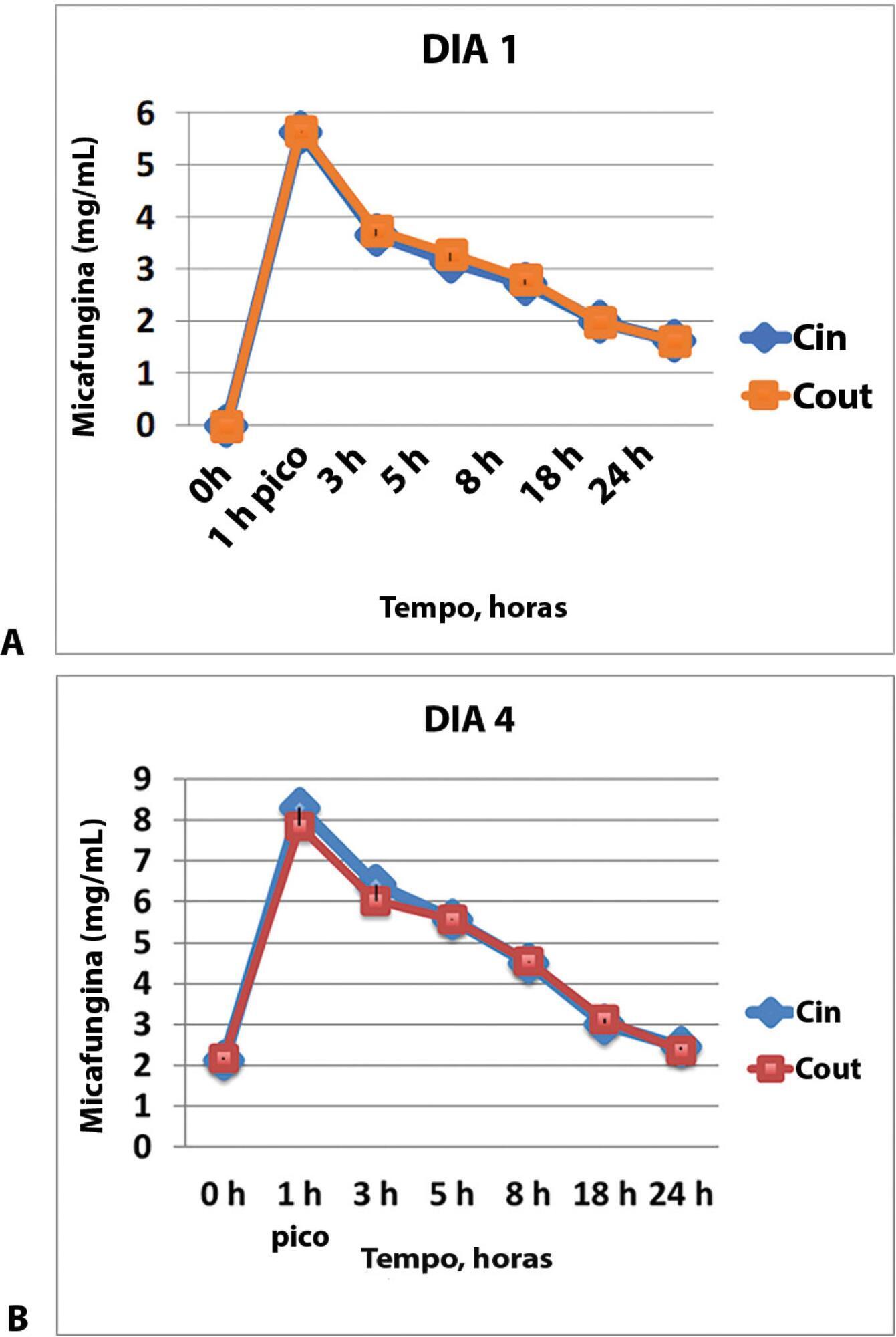
The samples were taken through an access point before and after the membrane in two tertiary hospitals in Spain. The times for the calculation of pharmacokinetic curves were before the administration of the drug and 1, 3, 5, 8, 18 and 24 hours after the beginning of the infusion on days one and four. The area under the curve, drug clearance, volume of distribution and plasma half-life time with a noncompartmental pharmacokinetic data analysis were calculated.
The pharmacokinetics of the values analyzed on the first and fourth day of treatment did not show any concentration difference between the samples taken before the membrane (Cin) and those taken after the membrane (Cout), and the pharmacokinetic behavior was similar with different organ failures. The area under the curve (AUC) before the membrane on day 1 was 62.1 (95%CI 52.8 – 73.4) and the AUC after the membrane on this day was 63.4 (95%CI 52.4 – 76.7), p = 0.625. The AUC before the membrane on day 4 was 102.4 (95%CI 84.7 – 142.8) and the AUC was 100.9 (95%CI 78.2 – 138.8), p = 0.843.
The pharmacokinetic parameters of micafungin were not significantly altered.
Search
Search in:


Comments